
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Help me with my study guide
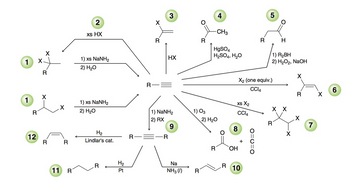
Transcribed Image Text:2
xs HX
1
X
1) xs NaNH2
2) H₂O
R
R
3
4
5
-CH3
R
R
H
HgSO4
H2SO4, H₂O
1) R₂BH
HX
X2 (one equiv.)
2) H2O2, NaOH
X
R
CCl4
6
R
X
X
1
X
R
2) H₂O
1) xs NaNH2
XS X2
X X
1) NaNH2
2) RX
1) O3
2) H₂O
CCl4
X
R
7
9
8
H2
12
R
R
R
R
Lindlar's cat.
+
R
OH
O=C=0
H₂
Na
R
R
10
11 R
Pt
NH3 (1)
R
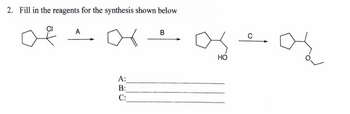
Transcribed Image Text:2. Fill in the reagents for the synthesis shown below
A
В
A:
B:
C:
ABC
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Devise concise syntheses for the following transformations. Clearly show the reagent andproduct for each step. All syntheses can be accomplished in two steps.arrow_forward1. Draw the major product of the reaction of 1-butanol and the following reagents. (a) PBr3 (b) SOCI2, py (c) HCI, ZNCI2 (d) Conc. H2SO4, heat (e) PCC, CH2CI2 (f) NażCr,O7, H2SO4, H2O (g) Li (h) NaH (i) TMSCI, Et3N (1) TSCI, pyridine (k) Na (1) Potassium tert-butoxidearrow_forwardI need help with the question attachedarrow_forward
- Acid-catalyzed hydrolysis of the following epoxide gives a trans diol. Of the two possible trans diols, only one is formed. How do you account for this stereoselectivity?arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardNicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forward
- Tamoxifen is a drug used in the treatment of breast cancer. How would you prepare tamoxifen from benzene, the following ketone, and any other reagents needed?arrow_forwardShow how the synthetic scheme developed in Problem 23.67 can be modified to synthesize this triiodobenzoic acid X-ray contrast agent.arrow_forward3. Provide a mechanism for the following reaction. OMe a) MeLi (2 equiv) b) H3O+ HO Me Mearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
