
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
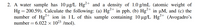
Transcribed Image Text:2. A water sample has 10 ug/L Hg²+ and a density of 1.0 g/mL (atomic weight of
Hg = 200.59). Calculate the following: (a) Hg²+ in ppb, (b) Hg+ in µM, and (c) the
number of Hg²+ ion in 1L of this sample containing 10 ug/L Hg²+ (Avogadro's
number
= 6.022 × 1023 /mol).
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student has a solution that contains 48.1 mg of PNP (MW = 139 g mol-1) in 650 mL of 45.0 % ethanol. Calculate the concentration of PNP in the solution (in µM).arrow_forward2. Using the data given below, calculate the percentage of KHP in the impure sample. The molar mass of KHP is 204.219 g/mol. Molarity of NaOH Solution: Mass of Unknown: Volume of Water Used to Dissolve Unknown: Volume of NaOH Used to Reach Equivalence Point: Percent of KHP: 0.1955 M 1.1801 g 30.00 mL 17.01 mL % Stolal Vol)arrow_forwardSolution X 100 mL of 0.10 M NaOH(aq) is mixed with 100 mL of 0.10 M HBr(aq) Solution Y 100 mL of 0.10 M NaBr(aq) is mixed with 100 mL of 0.10 M HBr(aq) Solution Z 100 mL of 0.10 M HC,H,O,(aq) is mixed with 100 mL of 0.10 M NaC,H3O,(aq) 6. A student prepares three solutions, X, Y, and Z, as described in the table above. The values of Ka for the acidic species in the solutions are given in the table below. Species Ka HBr(aq) >>I (very large) HC,H;O,(aq) 1.8 x 10-5 a. Using the information above, write the letters of the solutions in the boxes below to rank the solutions in order of increasing pH. Explain your reasoning for the ranking Lowest pH Highest pH b. Does the pH of solution Y increase, decrease, or remain the same when 100 mL of water is added? Justify your answer. c. The student adds 0.0010 mol of NaOH(s) to solution Y, and adds 0.0010 mol of NaOH(s) to solution Z. Assume that the volume of each solution does not change when the NaOH(s) is added. The pH of solution Y changes…arrow_forward
- How many grams of CaCl2 powder is need to dissolve in 100 mL water , if we only need 25% concentration of CaCl2?arrow_forwardPhosphate that is added to our drinking water forms a protective layer of lead phosphate on the inside of the pipes that distribute that water to our homes. The EPA maximum limit for lead in drinking water is 50ppb. Calculate the concentration of phosphate needed to keep the lead concentration below 39ppb. The Ksp of lead phosphate Pb3(PO4)2 is 1.6×10-32. Report your answer in ppb to the nearest whole number. Phosphate is 94.97 g/mole and lead is 207.2 g/mole.arrow_forwardQuestion 7 of 10 Submit What volume in L of a 0.724M Nal solution contains 0.405 | mol of Nal ? X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) %3D 0.405 0.293 g Nal L 126.90 g Nal/mol 0.559 mol Nal 559 mL 22.99 M Nal 148.89 6.022 x 1023 1.79 0.724 Tap here or pull up for additional resourcesarrow_forward
- Use the graph to rank these solutes in order of increasing solubility, lowest (1) and highest (4) at 10 °C. Grams of solute per 100 g H₂O 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 KI 1234 NH3 1234 NaNO3 KCIO Question 7 options: 1234 1234 KNO3 NHẠC Ce₂(SO4)3 0 0 10 20 30 40 50 60 70 80 90 100 Temperature (°C) KCI Naci KI KNO3 NaNO3 KCIO3 Iarrow_forwardo A chemist wants to extract a solute from 100 mL of water using only 300 mL of ether. The distribution constant, Kp, between ether and water is 1.99. Calculate q, the fraction of solute that would remain in the water under each of the extraction conditions. The chemist performs a single extraction with 300 mL of ether. 9 = The chemist performs three extractions with 100 mL of ether. 9= The chemist performs six extractions with 50 mL of ether. 9 =arrow_forwardA solution saturated in Mg(OH)2 is 1.12 x 10 M. Find Kgp of Mg(OH)2.arrow_forward
- Grams of solute / 100 g H,0 Suppose 100 g of NANO, 60°C. Using the solubility curves below, which term best 3 is dissolved in 100 g of water at describes this solution? Solubility Curves 150 140 KI 130 120 NANO, KNO 110 100 06 80 HCI NH.CI 70 60 KCI NH3 50 40 NaCI KCIO, 30 20 10 SO2 10 20 30 40 50 60 70 80 90 100arrow_forwardBa2 What is the molar concentration of present in 424 ppm of BaCl, -2H,0 (244.3 g/mol) 1.73×10-3 M 0.424 M More data is required to solve the problem 1.73 Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY