
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
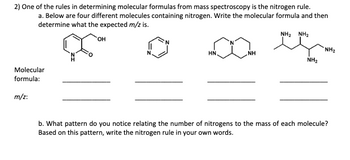
Transcribed Image Text:2) One of the rules in determining molecular formulas from mass spectroscopy is the nitrogen rule.
a. Below are four different molecules containing nitrogen. Write the molecular formula and then
determine what the expected m/z is.
Molecular
formula:
m/z:
OH
NH2 NH2
∞eç
NH
HN.
NH₂
NH₂
b. What pattern do you notice relating the number of nitrogens to the mass of each molecule?
Based on this pattern, write the nitrogen rule in your own words.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 27. The rule you generated in Question 14b for finding the [M] peak on the mass spectrum of an unknown molecule does not work very well if there is a Br or Cl in the molecule. Shown below are spectra of various compounds containing one Br or Cl. a. Find the [M] and [M+2]* peak on each spectrum. Relative Intensity 100- Relative Intensity 80- 60 20- 0-mmm 100- 80 60- 40 18--8163 20- 25 HS-NU-2127 0-forttitor 10 20 b. 30 50 40 50 Br pretztenyeregtelefongum 75 m/z 60 m/z Bri 70 100 (2) one Br atom. 125 effleyttim 90 80 ||||||| 100 110 Relative Intensity 100 Relative Intensity 80 60- 40 20 0-m 100- 80 60 40 20 15-MM-0968 0- spatepragning 25 115--1439 25 50 50 75 NH₂ 100 m/z mn||| 75 m/z 125 100 150 125 Write a rule for quickly identifying from a mass spectrum if an unknown molecule has... (1) one Cl atom. Note: The mass spectrum of a molecule with more than one Cl or Br can be quite complex, so we will limit cases to one Cl or Br. Write a new rule for finding the [M]* peak that takes into…arrow_forwardChemistry Instrumental Analysis Class The experiment is OES: Cleaning Validation of removal of pharmaceutical drugs and calibration standards by fluoresence spectroscopy - Why the instrument use two detectors and monochromators? What type of monochromators are used in our instrument? - Do you think that a similar experiment can be prepared for the analysis of naproxen and benzoic acid? Explain.arrow_forwardSample #1 has an absorbance of 0.300. Sample #2 is the same substance and has an absorbance of 0.600 using the same equipment. What is the relative concentration of the second sample? Select one: a. The concentration of sample #2 is the square root of that of #1. b. The concentration of sample #2 is that of sample #1 squared. c. The concentration of sample #2 is half that of sample #1. d. The concentration of sample #2 is twice that of sample #1.arrow_forward
- Which of the following formulae is consistent with a molecular ion of m/z 73 in a mass spectrometry experiment? O C3H8N2 O C4H11N O C4H10O O C3H5CIarrow_forwardDraw the molecular ion (M) for this molecule formed in the mass spectrometer. H IIZ: H -e Qarrow_forward1.Calculate the theoretical yield of acetylsalicylic acid from the given starting amount of salicylic acid. Show the calculation with units. Given: 1.4 g of your salicylic acid product in a 125 mL Erlenmeyer flask; record the weight Next, briefly explain if possible:What key change do you expect to see in the IR spectrum when the salicylic acid starting material is converted to the acetylsalicylic acid product?And or: What key change do you expect to see in the 1H NMR spectrum when the salicylic acid starting material is converted to the acetylsalicylic acid product?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY