
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:2. Draw the products of the reactions below, making sure to specify stereochemistry where applicable.
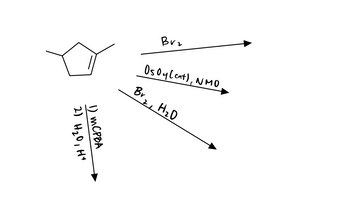
Transcribed Image Text:### Reaction Pathways of Cyclopentene
This image depicts various chemical reactions of cyclopentene, a five-membered carbon ring with a double bond.
1. **Reaction with Br₂ (Bromine)**
- When cyclopentene is treated with bromine (\( \text{Br}_2 \)), halogenation occurs. This reaction typically results in the addition of bromine atoms across the double bond, converting it into a dibromide.
2. **Osmylation with \( \text{OsO}_4 \)(Cat.), NMO**
- Osmium tetroxide (\( \text{OsO}_4 \)), in the presence of a catalytic amount, with N-Methylmorpholine N-oxide (NMO), results in the dihydroxylation of the alkene. This adds OH groups across the double bond of cyclopentene, forming a vicinal diol.
3. **Bromine in Water (\( \text{Br}_2, \text{H}_2\text{O} \))**
- Treating cyclopentene with bromine in the presence of water leads to bromohydrin formation. One bromine atom and one hydroxyl group are added across the double bond.
4. **Epoxidation and Hydrolysis**
- Using m-CPBA (meta-Chloroperoxybenzoic acid):
1. Epoxidation step forms an epoxide from the alkene.
2. Subsequent hydrolysis with water in acidic conditions results in the opening of the epoxide ring to give a diol.
This schematic helps illustrate the range of transformations possible with cyclopentene, showcasing its reactivity with different reagents and highlighting the versatile nature of alkene chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ex. 110 - Calculating Mass of 3 attempts left Check my work What mass of oxygen is needed to react with 1.79 gal of methanol according to the balanced equation below? (1.00 gal = 3.79 L, and the density of methanol is 0.793 g/mL.) 2 CH3OH() + 3 02g) – 2 CO2g) + 4 H2O(g) If appropriate, express your answer in scientific notion. (Click on the answer box to show the pallet.) < Prev 3 of 5 ***** ****arrow_forward(hemioi Appe fo ot fat Chemnty te bpe Question 19 ef 30 Determine the total mass of the product that 6ould be formed from the balanced reacion shown below if one starts with 4.20 gAl (26 98 g/mol) and 17.40 giodine, (MW 253 80 g/mol). 2AL# 31=2/All NEXT 7 Set up the table below that represents 100% yield with the given reaction conditions.. 2A1 2All Before (mol) Change (mol) After (mol) RESET 0456 0456 0.0606 0.0606 0.0457 00457 6468 0409 O10 0.093 0.068 # 0 > 面病和 type here to6 searcharrow_forwardHow many moles of NaOC1 would be present in 1 gallon of commercial bleach (density = 1.05 g/ml), which is 6.25% mass percent active ingredient. (1 L = 1.057 qt.)arrow_forward
- ||| A ALEKS-Katelyn Pearson... 1 Apromatincat Rom Fecr] ATOMS, IONS AND MOLECULES Predicting the formula of ionic compounds with common... Write the empirical formula for at least four ionic compounds that could be formed from the following ions: OH, Fe²+, 103, NH b Answered. The following X O Garrow_forwardAME M Inbox (1,600)-fantil@udeledu X Mail- Francesca A Tantillo-Out x Homepage - CHM150-251 Chen X + → Capp.101edu.co < A Z 89'1 Rain coming 2 S # 3 E D C Uranium hexafluoride, UF, is an important compound used in the enrichment of uranium by gaseous diffusion. $ Which of the following best describes where fluorine can be found on the periodic table? 4 40 R % F 5 B) Noble gases A) Alkaline earth metals C) Lanthanides D) Actinides E) Halogens G O Question 19.b of 23 6 B Aktiv Chemistry OME V Y H * الات & 7 -0✓ INN N PrtScn * 8 J Home End o M ( O 9 K O ) 0 L Alt Pgup m P Pon 12 B ? □ ( 4D Update ***/***/// 512 PM D 7/6/2022 < X Submit Del Backspace Enter Shiftarrow_forwardUse the acidity model pH = −log[H+], where acidity (pH) is a measure of the hydrogen ion concentration [H+] (measured in moles of hydrogen per liter) of a solution.Find the pH when [H+] = 4.7 × 10−5. (Round your answer to two decimal places.)pH =arrow_forward
- Propose a structural formula for the compound C,H6 and account for its formation. 1016 H 1. CH3I, 2 moles 2. Н,О 3. heat C10H16 H NH,arrow_forwardConsider the balanced reaction 7 A + 6 B → 3 C + 6 D. If 3.986 moles of A (MW = 35.44) are consumed, how many grams of D (MW = 35.02) are produced?arrow_forwardUsing the table provided, what is the enthalpy for the reaction shown below: 6H₂(g) + 4NO(g) + CH₂(g) → CO₂(g) + 2H₂O(l) + 4NH3 (9)arrow_forward
- (4b-101) Only using the list of ions below, form two ionic compounds that would be soluble in water, and two ionic compounds that would be insoluble in water. You should make four different compounds, but you may use ions more than once. Be sure to indicate which is which, and make sure your compounds are charge-balanced! Na+ Cour... Ca2+ Ni3+ NH4* s2- so42- Br OH For the toolbar, press ALT+F10 (PC) or ALT+FN+F10 (Mac). BIUS Paragraph Arial 14px A v Ix E = = E x² X2 次 T T ABC 田 田田图 - (;} Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answers Save and Submit APR 1600 4,104 Aa MacBook Air 80 000 O00 F1 F2 DII DD F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 @ 23 $ & ( ) 1 2 3 4 5 6 7 8 %3D delete Q W E R T Y { } 121 II 了E 田 * +] + 21arrow_forward요 Eto OEt 1. EtMgBr(xs) 2. H3O* HOarrow_forwardd) H3C CH 3 2 C₂H,MgBr H₂O*arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY