
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
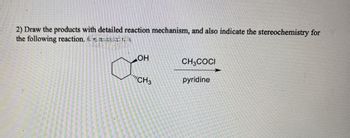
Transcribed Image Text:2) Draw the products with detailed reaction mechanism, and also indicate the stereochemistry for
the following reaction.
U
OH
CH3COCI
CH3
pyridine
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- The following isomers react separately with sodium hydroxide to give different products with the formulas shown. HO. .CI Но NaOH NaOH C3H1,02 (a) Draw the structure of each product. (b) Draw the mechanism that accounts for the formation of each of those products. (c) Explain why the isomeric reactants lead to different products.arrow_forwardProvide to llaving Br a detailed, step by step mechanism for the reaction OCH₂CH3₂ OCH₂CH3 OCH₂ CH3arrow_forwardGive detailed mechanism Solution with explanation needed..don't give Handwritten answerarrow_forward
- Give detailed mechanism Solution with explanation needed...don't give Handwritten answerarrow_forwardGive correct detailed Solution with explanation needed..don't give Handwritten answer....??arrow_forwardGive clear detailed mechanism Solution with explanation needed. don't give Ai generated solution.arrow_forward
- The following elimination reaction results in a mixture of products. Explain why the major products obtained in larger quantities than the minor products. Use chemical structures as necessary to justify your answer.arrow_forwardGive detailed mechanism Solution with explanation needed...please avoid handwritten Solutionarrow_forwardGive detailed Solution with explanation needed of each step....don't give Handwritten answerarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning