
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
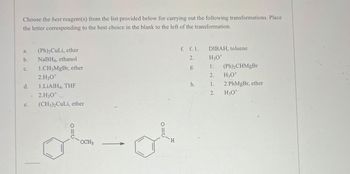
Transcribed Image Text:Choose the best reagent(s) from the list provided below for carrying out the following transformations. Place
the letter corresponding to the best choice in the blank to the left of the transformation.
a.
(Ph)2CuLi, ether
b.
NaBH4, ethanol
C.
1.CH3MgBr, ether
2.H3O+
d.
1.LiAlH4, THF
e.
2.H3O+
(CH3)2CuLi, ether
H
لی علی
OCH3
f. f. 1.
2.
DIBAH, toluene
H3O+
g.
1:
(Ph)2CHMgBr
2.
H3O+
h.
1.
2 PhMgBr, ether
2.
H3O+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Select reagents from the following table to bring about this conversion.arrow_forwardSpecify both the alcohol starting material and the reagents you would use in each step in a synthesis of the compound shown. If the synthesis requires only two steps enter "none" for step 3. Alcohol Starting Materials 1. methanol 2. ethanol 3. 1-propanol 4. 2-propanol 5. cyclohexanol Reagents available а. LIAIH4 f. PBr3 k. CH3 CH2 CH, MGB1; then H3O+ b. I. C,H5 MgBr (phenylmagnesium g. H2 SO4 CrO3, H,SO4, H,O bromide); then H30 + с. НСІ h. NaH m. (CH3)2 CHMGB.: then H3 O+ i. CH3MGB1; then H3O+ d. HBr n. Dess-Martin periodinane (Previous Nextarrow_forwardChoose the correct reagent or series of reagents from the ones listed to carry out the following synthesis. CH₂ O 1. 03.-78°C 2. Zn, CH3CO₂H O H₂O, H+ O CH₂OH, H+ OBH3, THF followed by H₂O₂, HO™ OH CH₂arrow_forward
- Which will complete the following reactions? Choices: A. HBr / ether B. PBr3 / ether C. SOCl2 / pyridine D. Br2 / NBS E. Chlorocyclopentane F. Cyclopentanol G. Iodobenzenearrow_forwardPart A Provide the major organic product(s) of the reaction below. H₂C CH 3 H3C H 1. LiAlH4 2. H¹, H₂Oarrow_forwardQuestion 14arrow_forward
- Examine the reaction/mechanism provided for the acid-catalyzed acetylation of an alcohol (ROH). Which step in this mechanism is incorrect? O 1 ΗΤΗ H 8 OR OH 2 O H-OH R-OH A. The deprotonation step from 3 to 4. OB. The protonation step from 4 to 5. OC. The nucleophilic attack step from 2 to 3. OD. All mechanism steps are correct. E. The protonation step from 1 to 2. 7 I OR + I 0-H O 20 RH 3 HO O 6 H-OH R R -O I 5 H 20-1 ΗΤΗarrow_forwardIdentify the correct reagents and conditions to perform this chemoselective reaction: H ရှုံး H Select one: Me e. Reagent(s) and conditions? a. NaBH4 b. BH3 THF Pd/BaSO4, H₂ (1 atm.), quinoline, Pb(OAC)2, MeOH d. Pd/C, H2 (1 atm.), MeOH DIBAL Me H H OHarrow_forwardWhich reagent(s) is/are needed to perform the following reaction (choose all that apply).CHOOSE ALL THAT APPLY a. H2O b. H2 c. Pt d. NaOH e. K2Cr2O7 f. H+ g. Cu^2+arrow_forward
- Suppose you are given the starting molecule shown below. For each of the cases listed below, list the reaction conditions and reagents that would give that product, and give a brief explanation of why ONLY the specified product would be formed under the conditions you chose. Make product alcohol with OH group at carbon 1. Make product alcohol with OH group at carbon 2. Make product alcohol with OH group at carbon 3. а. b. 3 2 C.arrow_forwardChoose the best reagents to complete the reaction shown below.arrow_forwardFrom the reaction conditions provided, design a four-step reaction sequence that is expected to convert the starting material into the product shown? For each step, only the major expected product can be taken forward. Assume standard workup as necessary. of 1. Cros, pyridine 2. BHs, THF, H2O2, H20 3. HBr 4. (COC2)2. MeOH 5. HBr, ROOR, heat 6. NBS, light 7. tBuONa, tBuOH 8. H2SO4, H2O Step 1: choose your answer. Step 2: choose your answer. v Step 3: choose your answer. Step 4: >arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY