
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Question
2. Classify each of the following as either
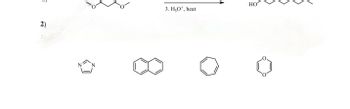
Transcribed Image Text:2)
3. H₂O, heat
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3.122 What type of reasoning were we using when we developed the equation for dilution, MiVi=MfVf ?arrow_forwardTungsten (W) metal, which is used to make incandescent bulb filaments, is produced by the reaction WO3+3H23H2O+W How many grams of H2 are needed to produce 1.00 g of W?arrow_forward6.) Hydrogen peroxide H2O2 is commonly used to clean bacteria in scrapes and cuts. It can be used as a mouthwash and is an additive in some toothpastes. If a sample contained 5.23 x 1021 molecules of H2O2 what would the equivalent be in moles? A) 8.69 x 10-3 mol B) 8.69 x 103 mol C) 115 mol D) 8.69 x 1043 molarrow_forward
- The semi-precious stone turquoise is a hydrate and with the chemical formula CuAls(PO4)-(OH);·4 H2O. a) How many aluminum atoms are in one formula unit of the hydrate? b) How many oxygen atoms are in one formula unit of the hydrate? c) What is the molar mass of the hydrate? d) What is the mass percent of aluminum in the hydrate? e) What is the mass percent of oxygen in the hydrate? f) What is the mass percent of water in the hydrate?arrow_forward1. Explain the term empirical formula and a molecular formula and state how they two differ. 2. Write the empirical formulas of the following: (a) H₂O₂= ; (b) C¿H= ; (c) C6H₁2O6 = ; (d) H₂0 = (e) MgO 12 NaOH g of O 3. Calculate the formula mass (a) NaCl = (b) KOH of Cu 9 = 4. What is the percentage of Ag in AgNO3 5. What is the percentage of C in C12H22O11 (Sucrose, Cane Sugar) 6. Determine in g the mass of 2.5 moles of = 7. How many moles in 0.5 g of Mg; and 0.2 of C₂H₂O₂. ; 8. Determine the number of atoms in 0.8 g 9. A substance was found by analysis to contain 45.57 % of Sn and 54.43 % of Cl. What is the empirical formula of the substance. 10. Ethylene Glycol has a molecular formula (a) Determine its molar mass (b) The percentage compositions of its component's elements (c) The empirical formula of Ethylene Glycolarrow_forwardGive me a clear handwritten answer with explanation..!!arrow_forward
- Please don't provide handwriting solutions....arrow_forwardWrite a balanced equation for the Alka Seltzer reaction where aqueous citric acid, H3C6H5O7, and aqueous sodium bicarbonate, NaHCO3, react and form aqueous sodium citrate, liquid water, and carbon dioxide gas.arrow_forwardMesitylene is a liquid hydrocarbon. Burning 0.795 g of the compound in oxygen gives 2.62 g of CO2 and 0.716 g of H2O. What is the empirical formula of mesitylene?arrow_forward
- 2.) 350 g of Calcium Chromate reacts with excess Hydrogen Peroxide. Write a balanced chemical equation and then calculate what mass of Calcium Peroxide will be formed.arrow_forward4. Write a balanced equation for the combustion of liquid methyl alcohol (CH3OH) in the presence of oxygen (O2), to produce carbon dioxide gas and gaseous water (dihydrogen oxide).arrow_forward2.Iron ore is impure Fe2O3. When Fe2O3 is heated with an excess of carbon (coke), metallic iron and carbon monoxide gas are produced. From a sample of ore weighing 938 kg, 523 kg of pure iron is obtained. What is the mass percent Fe2O3 by mass, in the ore sample, assuming that none of the impurities contain Fe?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning