
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A solution of acetone [(CH3)2C = O] in ethanol (CH3CH2OH) in the presence of a trace of acid was allowed to stand for several days, and a new compound of molecular formula C7H16O2 was formed. The IR spectrum showed only one major peak in the
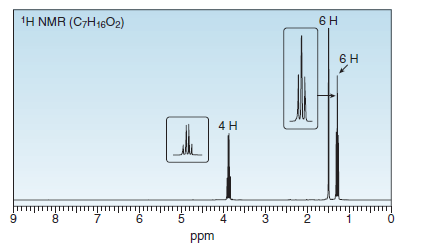
Transcribed Image Text:1H NMR (C7H1602)
4 H
8.
ppm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose the structure of the following alcohol? Molecular formula C5H12O The following peaks are found in proton NMR spectrum 0.91 δ (3H, triplet) 1.19 δ (6Η, singlet) 1.50 δ (2H, quartet) 2.0 δ (1H, singlet)arrow_forward1arrow_forwardCompound B of molecular formula C9H19N shows a noteworthy infrared absorption at 3300 cm-1. Its 1H-NMR spectrum shows three singlets – δ 1.0 (6H), 1.1 (12H), 1.4 (1H) ppm. Its 13C-NMR spectrum has four signals – δ 25, 28, 41, 64 ppm. Suggest a structure for this compound.arrow_forward
- Treatment of cis-4-bromocyclohexanol with HO− affords compound Aand cyclohex-3-en-1-ol. Treatment of trans-4-bromocyclohexanol under the same conditions forms compound B and cyclohex-3-en-1-ol. A and Bcontain different functional groups and are not isomers of each other.Propose structures for A and B and offer an explanation for theirformation.arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. ОН ОН p-TSCI CH,COO Na+ A 1. LIAIH, pyridine DMSO 2. H,О (R)-2-Octanol (S)-2-Octanol Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardOf the compounds shown below, the one numbered enamine H CH3 на CH3CH₂CHCH₂CH₂ 1 When a ketone reacts with 1 mole of alcohol, the resultant product is classified as acetal Ohemiacetal acetal hemiketal CH₂ Ph CHỊCH CHCH CH 2 CH₁ CH₂ حمله CH₂CH₂C=CHCH₂ 3 What is the best choice of reagent to perform the following transformation? Ph is an CIarrow_forward
- 3. What product would you get from the reaction between 2,4-dimethyl benzaldehyde with propylamine in the presence of H+? Establish the reaction and propose a reaction mechanismarrow_forwardPropose a mechanism for the conversion of E to F. The reagent used in this synthesis is ethyl chloroformate. The other product of this conversion is chloromethane, CH3Cl. Your mechanism should show how the CH3Cl is formed.arrow_forward1. There are several isomeric alkanes of molecular formula C6H14.Two of these exhibit the following 1H-NMR spectra. Propose a structure for each of the isomers. Isomer A: δ = 0.84 (d, 12 H), 1.39 (septet, 2H) ppm Isomer B: δ = 0.84 (t, 3 H), 0.86 (s, 9H), 1.22 (q, 2H) ppmarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY