
College Physics
1st Edition
ISBN: 9781938168000
Author: Paul Peter Urone, Roger Hinrichs
Publisher: OpenStax College
expand_more
expand_more
format_list_bulleted
Question
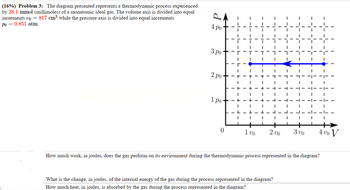
Transcribed Image Text:(16%) Problem 3: The diagram presented represents a thermodynamic process experienced
by 28.1 mmol (millimoles) of a monatomic ideal gas. The volume axis is divided into equal
increments vo=817 cm³ while the pressure axis is divided into equal increments
Po 0.851 atm.
4 po
-
3 po
2 po
1 po
-
+
+
+-
+
+
-1-
30
400 V
0
100
200
How much work, in joules, does the gas perform on its environment during the thermodynamic process represented in the diagram?
What is the change, in joules, of the internal energy of the gas during the process represented in the diagram?
How much heat, in joules, is absorbed by the gas during the process represented in the diagram?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A gas in a cylindrical closed container is adiabatically and quasi-statically expanded from a state A (3 MPa, 2 L) to a state B with volume of 6 L along the path 1.8pV= constant. (a) Plot the path in the pV plane. (b) Find the amount of work done by the gas and the change in the internal energy of the gas during the process.arrow_forwardOne mole of an ideal gas does 3 000 J of work on its surroundings as it expands isothermally to a final pressure of 1.00 atm and volume of 25.0 L. Determine (a) the initial volume and (b) the temperature of the gas.arrow_forwardA tank contains 111.0 g chlorine gas l2), which is at temperature 82.0 and absolute pressure 5.70105 Pa. The temperature of the air outside the tank is 20.0 . The molar mass of Cl2 is 70.9 g/mol. (a) What is the volume of the tank? (b) What is the internal energy of the gas? (c) What is the work done by the gas if the temperature and pressure inside the tank drop to 31.0 and 3.80105 Pa, respectively, due to a leak?arrow_forward
- Suppose 26.0 g of neon gas are stored in a tank at a temperature of 152C. (a) What is the temperature of the gas on the Kelvin scale? (See Section 10.2.) (b) How many moles of gas are in the tank? (See Section 10.4.) (c) What is the internal energy of the gas? (See Section 10.5.)arrow_forwardIn a cylinder of an automobile engine, immediately after combustion the gas is confined to a volume of 50.0 cm3 and has an initial pressure of 3.00 106 Pa. The piston moves outward to a final volume of 300 cm3, and the gas expands without energy transfer by heat, (a) What is the final pressure of the gas? (b) How much work is done by the gas in expanding?arrow_forwardIf a gas is compressed isothermally, which of the following statements is true? (a) Energy is transferred into the gas by heat. (b) No work is done on the gas. (c) The temperature of the gas increases, (d) The internal energy of the gas remains constant, (e) None of those statements is true.arrow_forward
- A sample of a monatomic ideal gas occupies 5.00 L at atmospheric pressure and 300 K (point A in Fig. P21.65). It is warmed at constant volume to 3.00 atm (point B). Then it is allowed to expand isothermally to 1.00 atm (point C) and at last compressed isobarically to its original state, (a) Find the number of moles in the sample. Find (b) the temperature at point B, (c) the temperature at point C, and (d) the volume at point C. (e) Now consider the processes A B, B C, and C A. Describe how to carry out each process experimentally, (f) Find Q, W, and Eint for each of the processes, (g) For the whole cycle A B C A, find Q, W, and Eint.arrow_forwardA 2.00-mol sample of a diatomic ideal gas expands slowly and adiabatically from a pressure of 5.00 atm and a volume of 12.0 L to a final volume of 30.0 L. (a) What is the final pressure of the gas? (b) What are the initial and final temperatures? Find (c) Q, (d) Eint, and (e) W for the gas during this process.arrow_forwardUnreasonable Results (a) How many moles per cubic meter of an ideal gas are there at a pressure of 1.001014N/m2 and at 0C ? (b) What is unreasonable about this result? (c) Which premise or assumption is responsible?arrow_forward
- (a) An ideal gas expands adiabatically from a volume of 2.0103 m3 to 2.5103 m3. If the initial pressure and temperature 5.0105 Pa and 300 K, respectively, what are the final pressure and temperature of the gas? Use =5/3 for the gas. (b) In an isothermal process, an ideal gas expands from a of 2.0103 m3 to 2.5103 m3. If the initial pressure and temperature were 5.0105 Pa and 300 K, respectively, what are the final pressure and temperature of the gas?arrow_forwardTwo moles of a monatomic ideal gas such as helium is compressed adiabatically and reversibly from a state (3 atm, 5 L) to a state with pressure 4 atm. (a) Find the volume and temperature of the final state. (b) Find the temperature of the initial state of the gas. (c) Find the work done by the gas in the process. (d) Find the change in internal energy of the gas in the process.arrow_forwardA mole of gas has isobaric expansion coefficient dV/dT=R/p and isochoric pressure-temperature coefficient dp/dT=p/T . Find the equation of state of the gas.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning