
Modern Physics
3rd Edition
ISBN: 9781111794378
Author: Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Use perturbation theory to calculate the first-order correction to the ground state energy of
an anharmonic oscillator whose potential energy is given by,
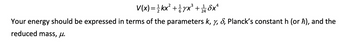
Transcribed Image Text:V(x) = 1½ kx² + yx³ + ¼ 1½ бx²
4
Your energy should be expressed in terms of the parameters k, y, &, Planck's constant h (or h), and the
reduced mass, μ.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 7 images

Knowledge Booster
Similar questions
- 1.2. Calculate the frequency, wavelength and momentum of a (a) 10 eV, and (b) 10 MeV photon of energyarrow_forwardWhat is the uncertainty in the temperature difference, ΔT, between the two temperature T1 = (25.0 ± 0.5) oC and T2 = (30.0 ± 0.3) oC?arrow_forward8πhy3 dv (a) Express the Planck radiation formula, Edv = c3 ehv/kT-1' terms of λ (and dλ), namely Edλ. (b) Solved=0 for λ = Amax in to determine the value of maxT in m-K, where max is the wavelength at which the blackbody spectrum has its maximum value at a given temperature T.arrow_forward
- Problem-1: An asteroid is hurtling toward earth at 150,000“. The temperature of the asteroid is about 100 K, meaning that its peak emission is 2 = 29 µm. The speed of light is c = 3E[8]. a) What is the wavelength of light that we receive from the asteroid? (Answer: 2.89855E[-05] m)arrow_forward4. In Section 1.3 we used dimensional analysis to show that the size of a hydrogen atom can be understood by assuming that the electron in the atom is wave-like and non-relativistic. In this problem we show that, if we assume the electron in the atom is a classical electron described by the theory of relativity, dimensional analysis gives an atomic size which is four orders of magnitude too small. Consider a relativistic, classical theory of an electron moving in the Coulomb potential of a proton. Such a theory only involves three physical constants: m, /4mc9, and e, the maximum velocity in relativity. Show that it is possible to construct a length from these three physical constants, but show that it too small to characterize the size of the atom.arrow_forward2.3. Find the de Broglie wavelength of (a) an electron, and (b) a proton with speeds of 5 × 106 m/s and compare with the radius of the hydrogen atom, ao. Would either of these particles behave like a wave inside the H atom?arrow_forward
- A photon in a laboratory experiment has an energy of 4.2 eV. What is the frequency of this photon? Planck’s constant is 6.63 × 10−34 J · s. Answer in units of Hz.arrow_forwardPlanck's principal assumption was that the energies of the electronic oscillators can have only the values &n=nhv and that Aε = hv. (a) Further assume that the number of oscillators with the energy En, Nn, is proportional to e-En/kT at the temperature T, namely Nn xe-En/kT, where N is the total number of oscillators. Show N that the average energy per oscillator is ɛ̃ = formula, Edv = formula as v → 0. ΣNnEn N c3 ehv/kT-1' = (b) As v→ 0, then Aɛ → 0 and & is essentially continuous. Hence, we should expect the non-classical Planck distribution to go over to the classical Rayleigh-Jeans distribution at low frequencies, where Ac→ 0. Show that the Planck radiation 8πhy3 dv reduces to the Rayleigh-Jeans hv ehv/kT-1arrow_forwardImagine another universe in which the value of Planck’s con- stant is 0.0663 J s, but in which the physical laws and all other physical constants are the same as in our universe. In this universe, two phys- ics students are playing catch. They are 12 m apart, and one throws a 0.25 kg ball directly toward the other with a speed of 6.0 m/s. (a) What is the uncertainty in the ball’s horizontal momentum, in a direction per- pendicular to that in which it is being thrown, if the student throwing the ball knows that it is located within a cube with volume 125 cm3 at the time she throws it? (b) By what horizontal distance could the ball miss the second students?arrow_forward
- A rectangular object has length l=30.3±0.6, width w=17.9±0.2. What is the absolute uncertainties in length?arrow_forward(prt 1)A photon emitted as a hydrogen atom undergoes a transition from the n = 4 state to then = 2 state.Find the energy of the emitted photon. Theenergy of the ground state is 13.6 eV.Answer in units of eV. (prt 2)Find the wavelength of the emitted photon.The speed of light is 3 × 108 m/s and Planck’sconstant is 6.626 × 10−34 J · s.Answer in units of nm. (prt 3)Find the frequency of the emitted photon.Answer in units of Hz.arrow_forwardFind the smallest possible uncertainty in the position of an electron moving with velocity 3x107 m/s. (Given: ħ= 1.054x10-34 Js, m. - 9.11 ×10-³1 kg) = Calculate 41 Jissarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning
Foundations of Astronomy (MindTap Course List)PhysicsISBN:9781337399920Author:Michael A. Seeds, Dana BackmanPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Modern Physics
Physics
ISBN:9781111794378
Author:Raymond A. Serway, Clement J. Moses, Curt A. Moyer
Publisher:Cengage Learning

Foundations of Astronomy (MindTap Course List)
Physics
ISBN:9781337399920
Author:Michael A. Seeds, Dana Backman
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning