
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
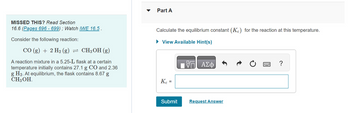
Transcribed Image Text:MISSED THIS? Read Section
16.6 (Pages 696 - 699); Watch IWE 16.5.
Consider the following reaction:
CO (g) + 2 H₂ (g) = CH3OH (g)
A reaction mixture in a 5.25-L flask at a certain
temperature initially contains 27.1 g CO and 2.36
g H₂. At equilibrium, the flask contains 8.67 g
CH3OH.
Part A
Calculate the equilibrium constant (Kc) for the reaction at this temperature.
► View Available Hint(s)
Kc =
Submit
ΑΣΦ
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose a 500. mL flask is filled with 0.30 mol of Br,, 1.5 mol of OCl, and 1.0 mol of BrOCl. The following reaction becomes possible: Br, (g) +OCl, (g) – BROC1(g) +BrC1(g) The equilibrium constant K for this reaction is 0.214 at the temperature of the flask. Calculate the equilibrium molarity of BrOCl. Round your answer to two decimal places. do Ar OM ?arrow_forwardAt a certain temperature, the equilibrium constant K for the following reaction is 6.0 x 10: co(e) + H,0(g) -co,() + H,(g) Use this information to complete the following table. Suppose a 31. L reaction vessel is filled with 1.1 mol of co, and 1.1 mol of H2. What can you say about the composition of the mixture in the vessel at equilibrium? O There will be very little CO and H,0. O There will be very little Co, and H,. O Neither of the above is true. What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K = ] Co,(0)+H,(0) CO()+H,O(9) 1. What is the equilibrium constant for the following reaction? Round your answer to 2 significant digits. K = ] 3 CO0g)+3H,0(9) 3 CO,(0)+3H,(9) 1.arrow_forwardSulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 2.0 L flask with 4.7 atm of sulfur dioxide gas and 0.67 atm of oxygen gas, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 0.80 atm. Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits. K = 0 P 0 x10arrow_forward
- A student ran the following reaction in the laboratory at 316 K:2CH2Cl2(g) CH4(g) + CCl4(g)When he introduced CH2Cl2(g) at a pressure of 0.487 atm into a 1.00 L evacuated container, he found the equilibrium partial pressure of CCl4(g) to be 0.219 atm. Calculate the equilibrium constant, Kp, he obtained for this reaction.arrow_forward6.arrow_forwardCarbon dioxide and water react to form methanol and oxygen, like this: 2 CO,(g)+4 H,O(g)→2 CH;OH(1)+3 O,(g) At a certain temperature, a chemist finds that a 4.6 L reaction vessel containing a mixture of carbon dioxide, water, methanol, and oxygen at equilibrium has the following composition: compound amount CO2 3.94 g H,0 4.50 g CH,OH 3.68 g O2 2.67 g Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. M K = 0 Lo B Cisc M Explanation Check A Chem 02022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility 9 Type here to search (99+ IA ASUS ZenBook Esc F2 F3 F4 F5 F6 F7 F8 %23 %24 21 4. * 6. L. R Y 3)arrow_forward
- Suppose a 500. mL flask is filled with 0.30 mol of Br₂, 1.2 mol of OC1, and 0.60 mol of BrCl. The following reaction becomes possible: Br₂(g) + OC1₂ (g) BrOCI(g) + BrCl(g) The equilibrium constant K for this reaction is 4.09 at the temperature of the flask. Calculate the equilibrium molarity of Br₂. Round your answer to two decimal places.arrow_forwardIn a particular experiment, it was found that when O,(g) and CO(g) were mixed and allowed to react according to the equation 2Co(g) + O2(3) = 2CO2(3) the O2 concentration had decreased by 0.062 mol Lwhen the reaction reached equilibrium. How had the concentrations of CO and CO2 changed? [CO] decrease = i mol/L [CO2] increase = mol/Larrow_forwardIn a particular experiment, it was found that when O2(g) and CO(g) were mixed and allowed to react according to the equation 2C0(g) + O2(g) 2CO2(g) the O2 concentration had decreased by 0.036 molL1when the reaction reached equilibrium. How had the concentrations of CO and CO2 changed? Incorrect. [CO] decrease = i ! mol/L Incorrect. [CO2] increase = i mol/Larrow_forward
- At a certain temperature, the reaction 2HF(g) H2lg) + F2lg) has K; = 1.2 × 1013. Does this reaction proceed far towards completion when equilibrium is reached? If 0.010 mol HF was placed in a 1.00 L container, and permitted to come to equilibrium, what would be the concentration of H2 and F2 in the container? Concentration of F2 at equilibrium - i M Concentration of H2 at equilibrium - i Marrow_forwardSulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 50.0 L tank with 15, mol of sulfur dioxide gas and 3.8 mol of oxygen gas, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 2.3 mol. Calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits. 0.P X CO M 00arrow_forwardA mixture of 0.100 mol of SO2 and 0.100 mol of O2 is placed in a reaction container and allowed to react until equilibrium is established. 2 SO2 (g) + O2 (g) -> 2 SO3 At equilibrium, 0.0916 mol of SO3 is present. a. What is the composition of the equilibrium mixture in terms of moles of each substance present? (Hint: Stoichiometry!) b. If the container size is 3.0 L, what is the value of the equilibrium constant?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY