
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Don’t show steps
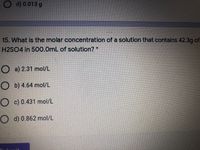
Transcribed Image Text:d) 0.013 g
15. What is the molar concentration of a solution that contains 42.3g of
H2SO4 in 500.0mL of solution? *
O a) 2.31 mol/L
O b) 4.64 mol/L
O c) 0.431 mol/L
O d) 0.862 mol/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- A gas sample is confined in a 4 L balloon. Which of the following will occur if the pressure on the balloon is increased at a constant temperature? I. The volume of the balloon will increase. II. The kinetic energy of the molecules of the gas in the balloon will decrease. III. The density of the gas will increase. a) I only b) || and III c) I and II d) |II only e) I, II, and IIarrow_forwardCan you do 1-10 pleasearrow_forwardCan you answer questions 7-10 please I don’t understand themarrow_forward
- As you breathe in, your diaphragm , increasing the volume of your lungs and the pressure in your lungs. As a result, the volume of the lungs will increase.arrow_forwardFinding vapor pressure / Partial pressure and moles using the Data collectionarrow_forwardStuden X F Jordan X Qjoin -0 X O SummX E Science X DOLSONguov deMkuCrNWWSbWQVN5vRLHXXJrULeM6va6T0vlo-fSg/viewform?hr_submission-DChk15se ebra II A-Ed. A Classes Your answer Consider the three flasks in the diagram below. What is the partial pressure of each gas and the total pressure after all the stopcocks are opened (in units of torr)? (latm = 760 torr) (760 torr = 101,325 Pa) Не Ne Ar 2.00 L 24,000 Pa 1.00 L 1.00 L 200 torr 0.4 atm Your answer DELLarrow_forward
- At STP, 23.50 mol of gas occupies approximately what volume in liters?arrow_forwardConvert 532 torr to kPa Remember that multiple steps may be required in the conversion. STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 1.01325 x 10° 1 1000 0.709 1.01325 0.700 101.325 0.001 105 14.70 760 0.98692 70.9 532 psi torr bar Pa mm Hg atm kPaarrow_forward#2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY