
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Question 5 of 9
Submit
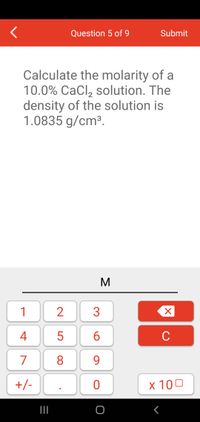
Calculate the molarity of a
10.0% CaCl, solution. The
density of the solution is
1.0835 g/cm³.
1
4
5
6
C
7
8
9.
+/-
x 100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the molality and molarity of an 85% by mass sulfuric acid (d=1.73 g/mL) solution.arrow_forwardCalculate the molarity of an aqueous solution that is 20.1% by mass calcium chloride. You might need to know that the density is 1.20 g/mLarrow_forwardFor each of the following solutions, calculate the molarity of the solution. 9.71 g MgNH4PO4 in 300. mL solution mol/L 12.6 g NaCH3COO in 500. mL solution mol/L 1.51 g CaC2O4 in 400. mL solution mol/L 4.96 g (NH4)2SO4 in 300. mL solution mol/Larrow_forward
- A student dissolves 15. g of ammonia (NH,) in 325. mL of a solvent with a density of 1.09 g/mL. The student notices that the volume of the solvent does not change when the ammonia dissolves in it. Calculate the molarity and molality of the student's solution. Be sure each of your answer entries has the correct number of significant digits. molarity molalityarrow_forwardCalculate the number of moles of solute present in 55.0 mg of an aqueous solution that is 1.40 m NaCl. Assume that for dilute aqueous solutions, the mass of the solvent is the mass of solution.arrow_forwardAn aqueous CaCl2 solution has a vapor pressure of 81.6 mmHg at 50 degrees celsius. The vapor pressure of pure water at this temperature is 92.6 mmHg. What is the concentration of CaCl2 in mass percent?arrow_forward
- A solution of KOH has 112 grams of KOH in 2000 grams of water. The Molality of this solution is O 230 O 28 O 4arrow_forwardDetermine the mass percent composition of a solution made by dissolving 55.6 g of KF in 898 g of H2O.arrow_forwardWine is 12% by volume ethanol (C2H5OH). What is the molarity of the solution? The density of ethanol is 0.789 g/mLarrow_forward
- Calculate the molarity of a solution which is 17.8% K2SO4·H2O by mass and has a density of 1.26 g/mL.arrow_forwardThe density of a solution that is 30.0 % H C l O3 is 1.167 g/mL. Calculate the molarity of the H C l O3 (molar mass is 84.46 g) solution.HINT : % H C l O3 = [mass H C l O3/mass solution] times 100% and Density = 1.167 g solution / 1 mL solution.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY