
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please help.
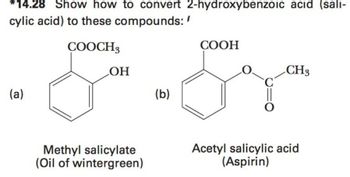
Transcribed Image Text:14.28 Show how to convert 2-hydroxybenzoic acid (sali-
cylic acid) to these compounds:/
COOCH3
(a)
OH
Methyl salicylate
(Oil of wintergreen)
(b)
COOH
CH3
Acetyl salicylic acid
(Aspirin)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O Relative intensity 100 50 15 mva 20 41 40 57 60 68 81 80 85 100 OH 1943. 120arrow_forward19-21arrow_forwardA+B+CD Exp. Initial A (mol/dm³) Initial B (mol/dm³) Init. Rate of Formation of D (mol/dm³ min-¹) 1 0.10 0.10 3.0 x 10-4 2 0.30 0.30 9.0 x 10-4 3 0.10 0.30 3.0 x 10-4 4 0.20 0.40 6.0 x 10-4 5 0.10 0.10 1.2 x 10-3 The student concluded that the rate equation should be: rate = K[A] [B][C]² ii) Using the data for experiment 4, calculate the value of the rate constant, k, using the correct rate equation, making sure that you include the correct units. Initial C (mol/dm³) 0.10 0.10 0.10 0.10 0.20arrow_forward
- How can we increase the stability of droplet in technical DSMEarrow_forward4PH3 P4 + 6H₂ What is the rate of disappearance of PH3 from 0 to 75 seconds? Time [PH₂] [P4] [H₂] 0 2.00 0 0 75 1.50 ? ? 150 1.25 0.19 1.13 225 1.13 0.22 1.31 300 1.07 0.23 1.40 RatepH3 [?] x 10 M/s Coefficient (green) Exponent (yellow) Enterarrow_forwardWhat is MRI? How does it work (5 sentences max)? And how much does it cost each time?arrow_forward
- The most likely route of entry for exposure to chemicals is through your nose and mouth. true falsearrow_forward3. Standard white vinegar you can buy in the grocery store is 5% concentration. That means 5% of the liquid vinegar is acetic acid and 95% of the solution is water. In a hardware store, you can buy industria strength vinegar, which is 30% concentration. This means that 30% of the vinegar is acetic acid, and the remaining 70% is water. Samuel does another experiment, this time with 5% vinegar and 30% vinegar. He sets up two science fair volcanoes (in no particular order), each with the same temperature, mass of baking soda and volume o vinegar. But one volcano uses 5% vinegar and the other volcano uses 30% vinegar. He measures the volume of gas production for the'first minute of each reaction, and he records the data below. Volcano # 1 Volume of gas produced (mL.) vs. Time (s) for Volcano #1 Time Volume of gas (s) produced (ml) 60 E 50 10 20 30 25 8 40 38 46 30 20 Volume of gas produced (ml) 40 50 50 60 52 of 10 53 20 40 60 80 Time (s) Volcano # 2 Volume of gas Volume of gas produced…arrow_forwardPlease find the rate and write rate law using given data.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY