
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
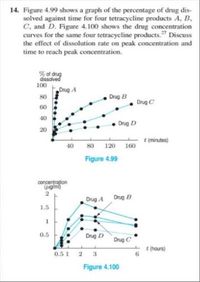
Transcribed Image Text:14. Figure 4.99 shows a graph of the percentage of drug dis-
solved against time for four tetracycline products A, B,
C, and D. Figure 4.100 shows the drug concentration
curves for the same four tetracycline products." Discuss
the effect of dissolution rate on peak concentration and
time to reach peak concentration.
% of drug
dissolved
100
Drug A
Drug B
80
Drug C
60
40
Drug D
20
t (minutes)
40 80 120
160
Figure 4.99
concentrațion
gml)
Drug A Drug B
1.5
0.5
Drug D
Drug C
t (hours)
0.51 2 3
Figure 4.100
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Methylethylketone (MEK) and water are partially miscible and hence form a mutually soluble two-phase system. R 298k, the MEK-rich layer contains 12% water and the water rich layer contains 25% MEK. If 100 kg each of water and MEK are mixed at 298K and allowed to reach equilibrium.• Calculate the weight of the MEK layer • Calculate the weight of the water layerarrow_forwardSOLVE STEP BY STEP DONT USE CHAT GPT In a laboratory, thymol blue (T) will be quantified using a calibration curve. To do this, a Stock solution of thymol blue Cstock =6 10^-5 M dissolved in 1 M HCI is prepared. From this solution, 5 systems are prepared (gauged with 1 M HCI), whose absorbance is read at 455 m, obtaining the following data: On the other hand, 2 mL was taken from a test solution and made up to 10 mL with 1 M HCI (solution A). Of this, 3 mL were taken and They made up to 10 mL with HCl (solution B). System B 1 Vstock (ml) 01 Vsoln B (ml) 00 Capacity (ml) 10/10 Absorbance 00.3512 T(M) 2 2 10 10 0.697 3 3 10 10 1.043 4 4 10 10 1.388 Determine the concentration of the test sample in system 6 Determine the concentration of thymol blue in the sample 5 5 lo 10 1.734 6 10 Fill in the table above with the concentrations of thymol blue in each of the systems on the curve. (7 decimal places) Based on the thymol blue concentrations in the calibration curve, determine the linear…arrow_forward3) A random poly(styrene-butadiene) copolymer has a number-average molecular weight of 350,000 g/mol and a degree of polymerization of 5000. Compute the fraction of styrene and butadiene repeat units in this copolymer.arrow_forward
- Which of these has the lowest first ionization energy? A. Al B. N C. Ne D. Rbarrow_forward2 attempts left Check my work Be sure to answer all parts. A solution contains 0.106 mol of NaCl dissolved in 7.47 mol of water. (a) What is the mole fraction of NaCl? (b) What is the molality of the solution? marrow_forwardConsider a diffusion couple composed of two semi-infinite solids of the same metal and that each side of the diffusion couple has a different concentration of the same elemental impurity; furthermore, assume each impurity level is constant throughout its side of the diffusion couple. For this situation, the solution to Fick’s second law) is as followsarrow_forward
- An aqueous 2-phase extraction method is used to purify a recombinant fusion peptide made in E. coli. To achieve the most efficient separation, 450 kg of a mixture of 19.7% w/w polyethylene glycol (PEG) and 17.7% w/w potassium phosphate salt in water is used. You notice there are some leftover solutions. You have: - 100 kg of a mixture of 20% w/w PEG in water - 150 kg of a mixture of 20% w/w PEG and 25% w/w salt in water Your supplies are the following: - 200 kg of an aqueous mix of 50% w/w PEG (stock solution) - 200 kg of an aqueous mix of 40% w/w salt (stock solution) - Unlimited water You’re an efficient engineer, so you’re going to use the leftover solutions first (waste not, want not, and all that). To get to that “most efficient separation,” how much of each stock solution and water is needed? You are going to use all of both leftover mixtures first.arrow_forwardQ5 in imagearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The