
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
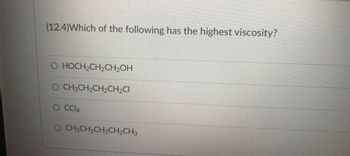
Transcribed Image Text:(12.4)Which of the following has the highest viscosity?
HOCH₂CH₂CH₂OH
O CH3CH₂CH₂CH₂Cl
CC14
O CH3CH₂CH₂CH₂CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Iridium (Ir) has a face-centered cubic unit cell with an edge length of 383.3 pm. What is the atomic radius of iridium? The atomic mass of iridium is 192.2 g/mol. (Avogadro’s #, NA = 6.022 x 1023/mol; 1 pm = 10–10 cm) (A) 135.5 pm (B) 166.0 pm (C) 191.6 pm (D) 271.0 pmarrow_forwardAluminum metal is produced by heating aluminumoxide, Al2O3(s), and other substances to almost1000 °C until the mixture melts. Molten aluminumoxide is an excellent conductor. An electric currentpassed through liquid aluminum oxide can providethe electrons needed to convert the aluminumions to neutral metal atoms. Explain the followingproperties of aluminum oxide in terms of ionicbonding: K/U(a) its high electrical conductivity when molten(b) its high melting pointarrow_forward15.26 How many grams of boiling water (at 100°C) must be added to a glass with 25.o g of ice at -3.0°C to obtain a liquid with a temperature of 45.0°C?arrow_forward
- For pairs of molecules in the gas phase, average H-bond dissociation energies are 17 kJ/mol for NH₃, 22 kJ/mol for H₂O,and 29 kJ/mol for HF. Explain this increase in H-bond strength.arrow_forwardHow do I rank these from strongest to weakest in terms of intermolecular forces (H-Bond, Dipole, London Dispersion)? Why would the ranking be this way?arrow_forwardHow do you solve this question?arrow_forward
- What amount of heat , in kj, is required to vaporized 190.55 g of ethanol (C2H5OH)?(Hvsp=43.3 KJ/mol)arrow_forwardAt room temperature, F2 and Cl2 are gases, Br2 is a liquid, and I2 is solid. This is because of what?arrow_forwardRank the following compounds in order of increasing surface tension at a given temperature:(a) CH3CH2CH2OH(b) HOCH2CH(OH)CH2OH(c) HOCH2CH2OHb < a < cc < b < aa < c < ba < b < cb < c < ac < a < barrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY