
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
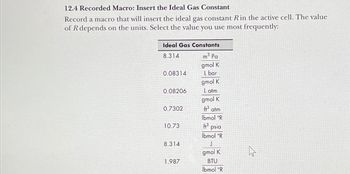
Transcribed Image Text:12.4 Recorded Macro: Insert the Ideal Gas Constant
Record a macro that will insert the ideal gas constant R in the active cell. The value
of R depends on the units. Select the value you use most frequently:
Ideal Gas Constants
8.314
m³ Pa
gmol K
0.08314
L bar
gmol K
0.08206
Latm
gmol K
0.7302
ft³ atm
lbmol °R
10.73
3 psia
lbmol °R
8.314
J
gmol K
1.987
BTU
lbmol °R
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider a cell reaction at 25°C where n=4 . Fill in the following table.arrow_forwardUse the following half-equations to write three spontaneous reactions. Justify your answers by calculating E° for the cells. 1. MnO4(aq)+8H+(aq)+5eMn2+(aq)+4H2OE=+1.512V 2. O2(g)+4H+(aq)+4e2H2OE=+1.229V 3. Co2+(aq)+2eCo(s)E=0.282Varrow_forwarda Calculate G for the following cell reaction: Tl(s)Tl+(aq)Pb2+(aq)Pb(s) The Gf for Tl+(aq) is 32.4 kJ/mol. b From G, calculate the standard cell potential for the cell reaction and from this, determine the standard potential for Tl2+(aq)+eTl(s).arrow_forward
- Consider a concentration cell that has both electrodes made of some metal M. Solution A in one compartment of the cell contains 1.0 M M2+. Solution B in the other cell compartment has a volume of 1.00 L. At the beginning of the experiment 0.0100 mole of M(NO3)2 and 0.0100 mole of Na2SO4 are dissolved in solution B (ignore volume changes), where the reaction M2+(aq)+SO42(aq)MSO4(s) occurs. For this reaction equilibrium is rapidly established, whereupon the cell potential is found to be 0.44 V at 25C. Assume that the process M2++2eM has a standard reduction potential of 0.31 V and that no other redox process occurs in the cell. Calculate the value of Ksp for MSO4(s) at 25C.arrow_forwardFor the standard cell potentials given here, determine the ?G for the cell in k].. (a) 0.000V,n=2 (b) +0.434V,n=2 (c) -2.439 V, n = 1arrow_forwardGive the notation for a voltaic cell whose overall cell reaction is Mg(s)+2Ag+(aq)Mg2+(aq)+2Ag(s) What are the half-cell reactions? Label them as anode or cathode reactions. What is the standard cell potential of this cell?arrow_forward
- The mass of three different metal electrodes, each from a different galvanic cell, were determined before and after the current generated by the oxidation-reduction reaction in each cell was allowed to flow for a few minutes. The first metal electrode, given the label A, was found to have increased in mass; the second metal electrode, given the label B, did not change in mass; and the third metal electrode, given the label C, was found to have lost mass. Make an educated guess as to which electrodes were active and which were inert electrodes, and which were anode(s) and which were the cathode(s).arrow_forwardYou have 1.0 M solutions of Al(NO3)3 and AgNO3 along with Al and Ag electrodes to construct a voltaic cell. The salt bridge contains a saturated solution of KCl. Complete the picture associated with this problem by a writing the symbols of the elements and ions in the appropriate areas (both solutions and electrodes). b identifying the anode and cathode. c indicating the direction of electron flow through the external circuit. d indicating the cell potential (assume standard conditions, with no current flowing). e writing the appropriate half-reaction under each of the containers. f indicating the direction of ion flow in the salt bridge. g identifying the species undergoing oxidation and reduction. h writing the balanced overall reaction for the cell.arrow_forwardIf a logarithmic scale had not been used for the graph of Figure 13.13, what would the plots look like? FIGURE 13.13 The variation of equilibrium constant with cell potential is shown. The different lines correspond to reactions involving the transfer of one, two, or three electrons, as indicated.arrow_forward
- Use the data from the table of standard reduction potentials in Appendix H to calculate the standard potential of the cell based on each of the following reactions. In each case, state whether the reaction proceeds spontaneously as written or spontaneously in the reverse direction under standard-state conditions. (a) H2(g)+Cl2(g)2H+(aq)+2Cl(aq) (b) Al3+(aq)+3Cr2+(aq)Al(s)+3Cr3+(aq) (c) Fe2+(aq)+Ag+(aq)Fe3+(aq)+Ag(s)arrow_forwardHow does galvanic corrosion differ from uniform corrosion of iron?arrow_forwardCalculate the theoretical potential of each of the following cells.Is the cell reaction spontaneous as written or spontaneous in the opposite direction? (a) Bi|BiO+ (0.0300 M),H+ (0.100 M)||I- (0.100 M), AgI(sat’d)|Ag (b) Zn|Zn2+(5.75 10-4M)||Fe(CN)64-(530 10-2 M),Fe(CN)63-(6.75 10-2M)|Pt (c) Pt,H2O (0.200 atm)|HCI(8.25 10-4M), AgCI(sat’d)| Agarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning