
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
** Although the answer is there, please explain how to do the problem!!
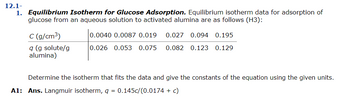
Transcribed Image Text:12.1-
1. Equilibrium Isotherm for Glucose Adsorption. Equilibrium isotherm data for adsorption of
glucose from an aqueous solution to activated alumina are as follows (H3):
C (g/cm³)
q (g solute/g
alumina)
0.0040 0.0087 0.019 0.027 0.094 0.195
0.026 0.053 0.075 0.082 0.123 0.129
Determine the isotherm that fits the data and give the constants of the equation using the given units.
A1: Ans. Langmuir isotherm, q = 0.145c/(0.0174 + c)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- I apologize upfront, but I do not understand the solutions here. I cannot follow along due to the way it is typed. I've tried writing it out many times and never come to the same answers as you. Is it possible to get these solutions hand written?arrow_forwardit's only one answer. each choice is to fill in both blanks. Not sure if b is the optimal choice between b and darrow_forwardWhat is activation energy? Please explain the equation of activation energy. How to measure the activation energy?arrow_forward
- I just got confused by this table isn't the enthalpy supposed to go lower in (highlighted)value how did he put it 130 then 400 ? Is there trick behind it. Please answer this queryarrow_forwardExplain why the potential energy release from a reactor accident is not com- parable to that of a nuclear explosive device. Identify the most significant and two other differences between a nuclear reactor and an explosive device. NOTEarrow_forwardWhich element has the electron configuration 1s 2s2p 3s²3p 4s'3d#? A) V B) Cr C) Мо 2) Mn E) No element has this configurationarrow_forward
- What is the Joule experiment? Why is it important? What does it prove?arrow_forwardPatm Fluid 1 = Oil, P1 = 790 kg / m, h1 = 50 cm Fluid 2 = Chemical X, p2 = 850 kg / m3, h2 = 120 cm Fluid 3 = Water, p3 = 1000 kg / mở, h3 = 80 cm %3D Fluid 1 h1 If atmospheric pressure is 101.3 kPa determine the absolute pressure, P at each of the following locations : 3 |kPa | kPa |kPa P@ Point 4 = Fluid 2 P@ Point 3 = h2 P@ Point 2 = КРа P@ Point 1 = Fluid 3 hzarrow_forwardHi! Can someone answer Learning task 4, no. 2? Thanks!arrow_forward
- Your Question :Your Question :Your Question :Your Question :help please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forwardRemaining Time: 20 minutes, 12 seconds. * Question Completion Status: QUESTION 1 The dimensions of heat flux is ML2T -3 True False QUESTION 2 Dimensionless parameters are obtained using a method called dimensional analysis. O True ロFalse QUESTION 3 Click Save and Submit to save and submit. Click Save All Answers to save all answers. earcharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The