
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
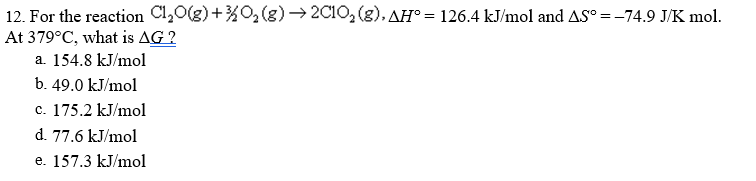
Transcribed Image Text:12. For the reaction Cl,0(g)+%O2(g) → 2C10, (g), AH° = 126.4 kJ/mol and AS° =-74.9 J/K mol.
At 379°C, what is AG ?
a. 154.8 kJ/mol
b. 49.0 kJ/mol
c. 175.2 kJ/mol
d. 77.6 kJ/mol
e. 157.3 kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Similar questions
- For a certain process at 455 K, A G=-12.1 kJ and A H=-9.2 kJ. Therefore, A S for the Oa. 6.37 J/K mol process is Ob.0 J/K mol Oc. 3.98 J/K mol d. 8.2 J/K mol O e.-8.2 J/K molarrow_forwardConsider the following reaction: SO((g) + CaO(s) = CasO4(s). If AGº is -345 kJ/mol for this reaction at 298K, what is the Keg at this temperature? Recall that R is 8.314 J mol-1 K-1. A) 3.1 x 1040 B) 3.50 x 1050 c) 2.96 x 1060 D 2.23 x 1070arrow_forwardHint: No calculations are required. For the reaction 2HBr(g) + Cl2(g)- >2HCI(g) + Br2(g) AH° = -81.1 kJ and AS° =-1.20 J/K At standard conditions, this reaction would be product favored O at all temperatures. O at relatively high temperatures. O at no temperature. O at relatively low temperatures.arrow_forward
- A certain liquid has a vapor pressure of 92.0 Torr at 23.0 °C and 259.0 Torr at 45.0 °C. Calculate the value of AHap for this liquid. AH vap = Calculate tl x10 boiling point: TOOLS s liquid. kJ/mol °Carrow_forwardWithout doing any calculations or using Sf data, predict whether the DS for each process would be positive or negative. A. Si(s) + 2Cl2(g) SiCl4(g)B. 2HgO(s) 2Hg(l) + O2(g)C. C12H22O11(aq) C12H22O11(s)arrow_forwardBelow is the reaction for the evaporation of water. H2O (I) H20 (g) AH°f H2O (1)= -285.8 kJ/mole ; H20 (g) = -241.8 kJ/mole %3D Calculate AH*rxn for this process. This is the reaction that happens when your sweat evaporates. Why is this a cooling mechanism for your body? How much sweat would have to evaporate from a person's skin to cool the body by 0.5 C? Assume this person has a body mass of 150 Ibs and the specific heat capacity of the body is 4.0 J/g °C. 1 Ib. = 0.454 kgarrow_forward
- Determine the AU°reaction at 25 °C (in kJ/mol) for the following reaction: 2NO(g) + O2(g)→2NO2(g) Appendix D kJ/molarrow_forward1. A reaction with AH° = -64.0 kJ/ mole has K = 1.00 X 108 at 25.0 °C. What would be the value of K at 0.00 °C? 2. A reaction with AH° = 618.0 kJ/mole has K = 1.00 X 1060 at 25.0 °C. What would be the value of K at 500.0 °C?arrow_forwardwhat letter choice is thisarrow_forward
- Calculate H°rxnfor the following reaction and the given H°f of the reactants and products. A. 440 kJ B. -2440 kJ C. -440 kJ D. 1960 kJarrow_forwardConsider the following reaction: H2(g)+I2(g)-->2HI(g) Given the following data: H2: delta H*= 0 kJ/mol; S*= 131 J/mol K I2: delta H*= 0 kJ/mol; S*= 104 J/mol K HI: delta H*= 25 kJ/mol; S*= 206 J/mol K Determine the delta G* in kJ for this reaction at 298K.arrow_forwardThe value of AH° for the reaction below is -336 kJ. Calculate the heat (kJ) released to the surroundings when 23.0 g of HCl is formed. 26. CH4 (g) + 3Cl, (g) → CHC1; (1) + 3HC1 (g) A) B) C) 211 177 70.7 D) -336arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY