
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
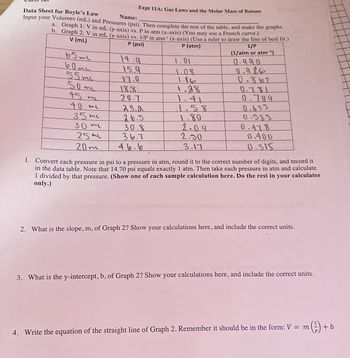
Transcribed Image Text:Expt 11A: Gas Laws and the Molar Mass of Butane
Data Sheet for Boyle's Law
Name:
Input your Volumes (mL) and Pressures (psi). Then complete the rest of the table, and make the graphs.
a. Graph 1: V in mL (y-axis) vs. P in atm (x-axis) (You may use a French curve.)
b. Graph 2: V in mL (y-axis) vs. 1/P in atm¹ (x-axis) (Use a ruler to draw the line of best fit.)
V (ml)
1/P
P (psi)
P (atm)
(1/atm or atm-¹)
65ml
60me
55 m
50ml
45 m
40 me
35 mi
30 ml
25 ml
20 ml
14.q
15.9
17.0
18.8
20.7
23.2
26.5
30.8
36.7
46.6
1.01
1.08
1.16
1.28
1.41
1.58
1.80
2.09
2.50
3.17
0.990
0.926
0.862
0.781
0.709
0.635
0.555
0.478
0.400
0.315
1. Convert each pressure in psi to a pressure in atm, round it to the correct number of digits, and record it
in the data table. Note that 14.70 psi equals exactly 1 atm. Then take each pressure in atm and calculate
1 divided by that pressure. (Show one of each sample calculation here. Do the rest in your calculator
only.)
2. What is the slope, m, of Graph 2? Show your calculations here, and include the correct units.
3. What is the y-intercept, b, of Graph 2? Show your calculations here, and include the correct units.
4. Write the equation of the straight line of Graph 2. Remember it should be in the form: V = m
(²) + b
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A gas sample containing 12.0 g of He has a volume of 14.0 L. What will the volume be if 2.4 g of H2 is added? (Temperature and pressure do not change.)arrow_forwardActivity 4: Graph Analysis Direction: Based on the given graph, analyze and infer the relationship of the properties of gases (volume, pressure, temperature, and moles). Write your answer at the sides of the graph. 1. Pressure, P 9. Temperature T (K) 3. Number of moles, n 2. Pressure P Volume,'arrow_forward16carrow_forward
- In Dalton's Law of Partial Pressures: Ptrapped gas = Pdry gas + Pwater vapor Why must the pressure of water vapor be included in this calculation? a. The pressure exerted by the evolved H2(g) is reduced because of the co-presence of water vapor, thus Pwater vapor has to be added to Pdry gas. b. Some of the liquid water evaporates into the gas, and increases the total pressure generated by the trapped gas. c. As the H2(g) is bubbled up the reaction solution, some of the H2(g) molecules are solubilized by water in the aqueous solution, effectively decreasing the measured pressure above. d. The pressure contributed by the H2(g) is greater than the measured total pressure because of the contribution of partial pressure of water vapor.arrow_forwardIf 0.211 g of gas occupies 204.5 mL at STP, what is the molecular weight of this gas? R = 0.08206 atm L / mole Karrow_forwardAt 570 mm Hg and 25.0 celcius a gas sample has a volume of 2270 ML. What is the final pressure (in mm Hg) at a volume of 1250 and a temp of 280 celsius?arrow_forward
- A.) a mixture of argon and neon gases, in a 8.32 L flask at 21 degrees Celsius, contains 15.3 grams of argon and 2.24 grams of neon. The partial pressure of neon in the flask is ____atm and the total pressure in the flask is ____atm. B.) a mixture of helium and xenon gases is maintained in a 9.17 L flask at a pressure of 1.28 atm and a temperature of 84 degrees Celsius. If the gas mixture contains 0.637 grams of helium, the number of grams of xenon in the mixture is ____grams.arrow_forwardP2=_____atmarrow_forwardAt 571 mm Hg and 323 K, a gas sample has a volume of 2.45 mL. What is the final pressure (in mm Hg) at a volume of 1.53 mL and temperature of 463 K? (Report your answer to the nearest 1 mm Hg)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY