
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please don't provide handwriting solution
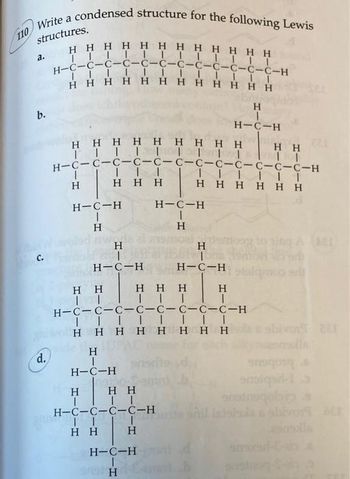
Transcribed Image Text:structures.
Write a condensed structure for the following Lewis
Η Η Η Η Η Η Η Η Η Η Η Η
|||||||||||II
H=C=C=C=C=C=C=C=C=C=C=C-C-H
IIIIIIII
Η Η Η Η Η Η Η Η Η Η Η Η
110
το
b.
d.
Η Η Η Η Η Η Η Η Η
ΤΤΤΤΙΤΤΤΤ
H=C=C=C=C=C=C=C=C=C-C-C-C-H
|
Η
1
H=C-H
|
H
Η Η Η
Η Η
II
H
|
Η
|
H=C-H
Η Η
ΤΙ
H=C=C-C-C-H
ΓΙ I
Η
Η Η
Η Η Η
Τ..
H=C-H
Η
H=C-H
|
ΤΗ
Η
|
H=C-H
Η
H=C-H
Τ.....
Η Η Η Η Η Η
H-C-C-C-C-C-C-C-C-H
ΤΤΤΙ....
Η Η Η Η Η Η Η Η
Η
H-C-H
Η
Η Η
ΤΤ
ΜΙΑ
στο
snadnoqolbypa
shivor acr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Convert the Lewis structure below into a skeletal structure. H H-C-H H S. H I | | | | н нн :0: Н Н H-C-C -C=C-C-C-C-C-H H H H C-H Harrow_forwardWrite the systematic name of each organic molecule: || structure H-C CH2 CH21 CH- - CH3 OH O= CH3–CH−C−H | CH3 || H-C-CH2-CH2-CH-CH3 CH3 name ☑arrow_forwardUsing average bond enthalpies (linked above), estimate the enthalpy change for the following reaction: 2C0(g) + 02(g) 2CO2(g)arrow_forward
- un muinsup to 2192 griwellol and to riass of brot 91 fals 9. For the following skeletal structures, add the appropriate bonds and nonbonding electrons so each atom will have zero formal charge and a filled octet with the exception of hydrogen. H—C—C—C—C-H12-ais H H H—C—C-C-C-H | | | H H H H H y H C-CH F C woll (d W 2 on sa (darrow_forwardWrite in any formal charges not equal to zero. If there are none, please check the box below. :0 A H-C-C-H | | H H ☐ There are no missing formal charges. !arrow_forwardOrder the following carbon-carbon bonds in order of increasing vibrational frequency (lowest to highest in cm-1) I C=C 0 0 0 0 | < ||| < || || < ||| < | || < | < ||| ||| < || < | II C-C III C=Carrow_forward
- Convert the following line-bond structures into molecular formulas: (a) CH₂OH H H H H کی C= = с с C )-1 H (c) H、 N. с (=^ 0=C с FO Aspirin (acetylsalicylic acid) 0 )=0 с C C=0 OH H C || H CH3 ć с N Nicotine C 1 с с H HY C-C T H H CH3 )—I C H I I H (b) (d) HO H 4-C I HO HO H H₂ H-C C=0 Vitamin C (ascorbic acid) C=C CH₂OH C-O C C-C HO но| | H H OH OH Glucose H OH 11arrow_forwardPROVISIONAL LEWIS STRUCTURES T01/S02 For each provisional structure, enter the central atom formal charge (if not zero, enter the sign before the value) and, separating by comma without any space, indicate (Y or N) if it is the correct Lewis structure. For example, your answer could be "-2,Y". :ä: H - C -H | CH2CI2 BrF2+ :o: H-ö-B Н — Ве— н :o: B(OH)4 BeH2arrow_forward||| = O PRINCIPLES OF ORGANIC CHEMISTRY Identifying rigid parts of an acyclic organic molecule In the actual molecule of which this is a Lewis structure, which of the labeled distances can change? H marked O G: F H B C H A N. unmarked G с N O H 0,0.... List all the distances that can change. For example, suppose all the distances were measured at a certain time, and again 0.1s later. If distance A might be 50% bigger or smaller the second time, but all the other distances are certain to be the same, you should write "A". If A and B might be different the second time, but no other distances, you would write "A, B". And so on. Note for advanced students: you can assume the molecule is dissolved in an appropriate solvent at room temperature. X 0/5 You can click the "unmarked" tab to see the molecule without any of the distances marked. S Jacari V 18 CO olo Ararrow_forward
- MOLECULES Drawing a skeletal structure from a Lewis structure Convert the Lewis structure below into a skeletal structure. H H-C-H H :0: || -N-C=ċ-c-0-C-H H Н-С—С- H H Н—С—Н Н Harrow_forwardDetermine the formal charge on the nitrogen atom in each of the structures. NH3 NH2 ート N. H. H. H2N-OH C F G All nonbonding electrons are shown. What is the formal charge on the nitrogen atom in What is the formal charge on the nitrogen atom in structure A? structure B? 00 00 :O: :0=z :O:arrow_forwardThe bond dissociation energy to break 9 C-H bond(s) in 1 mole of hexane (CH₃CH₂CH₂CH₂CH₂CH₃) molecules isarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning