
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please answer and show all work for parts A-C, thank you!! Thermodynamics table is shown below :)
![### Educational Content on Thermodynamics
#### Reaction Analysis of Methane Combustion
**Reaction:**
\[ \text{CH}_4(g) + 2 \text{O}_2(g) \rightarrow \text{CO}_2(g) + 2 \text{H}_2\text{O}(l) \]
**Entropy Change:**
\[ \Delta S = -154 \, \text{J/K} \]
**Questions and Solutions:**
**a. At what temperature does this reaction begin to occur spontaneously?**
*Hint:* Use the Thermodynamic Tables.
To determine the temperature at which the reaction becomes spontaneous, you should use the Gibbs Free Energy equation:
\[ \Delta G = \Delta H - T\Delta S \]
Where \(\Delta G\) must be negative for spontaneity.
**b. Is the reaction spontaneous at 50°C? Show your work.**
To check if the reaction is spontaneous at 50°C (which is 323K), calculate \(\Delta G\) using the known \(\Delta H\) and \(\Delta S\). If \(\Delta G < 0\), the reaction is spontaneous.
**c. Calculate the Joules of work done on or by the system when 1 mole of CH\(_4\) is combusted at 127°C.**
*Hint:* R = 8.31 J/mol K
To calculate the work done, analyze the system's expansion or compression work, often associated with changes in volume and pressure. You may need to determine \(\Delta G\) at 127°C (400K) and use the ideal gas constant \(R\) to find this work.
---
**Note:** Detailed calculations would involve accessing thermodynamic tables for specific values like enthalpy (\(\Delta H\)) and applying them to the formulas provided.](https://content.bartleby.com/qna-images/question/17525826-644e-4b54-80a2-4cc5c7d853ae/51004991-3d29-4abd-945b-790572699edf/urgilqa_thumbnail.jpeg)
Transcribed Image Text:### Educational Content on Thermodynamics
#### Reaction Analysis of Methane Combustion
**Reaction:**
\[ \text{CH}_4(g) + 2 \text{O}_2(g) \rightarrow \text{CO}_2(g) + 2 \text{H}_2\text{O}(l) \]
**Entropy Change:**
\[ \Delta S = -154 \, \text{J/K} \]
**Questions and Solutions:**
**a. At what temperature does this reaction begin to occur spontaneously?**
*Hint:* Use the Thermodynamic Tables.
To determine the temperature at which the reaction becomes spontaneous, you should use the Gibbs Free Energy equation:
\[ \Delta G = \Delta H - T\Delta S \]
Where \(\Delta G\) must be negative for spontaneity.
**b. Is the reaction spontaneous at 50°C? Show your work.**
To check if the reaction is spontaneous at 50°C (which is 323K), calculate \(\Delta G\) using the known \(\Delta H\) and \(\Delta S\). If \(\Delta G < 0\), the reaction is spontaneous.
**c. Calculate the Joules of work done on or by the system when 1 mole of CH\(_4\) is combusted at 127°C.**
*Hint:* R = 8.31 J/mol K
To calculate the work done, analyze the system's expansion or compression work, often associated with changes in volume and pressure. You may need to determine \(\Delta G\) at 127°C (400K) and use the ideal gas constant \(R\) to find this work.
---
**Note:** Detailed calculations would involve accessing thermodynamic tables for specific values like enthalpy (\(\Delta H\)) and applying them to the formulas provided.
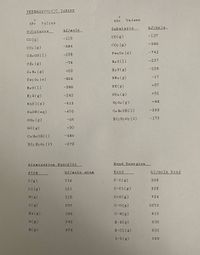
Transcribed Image Text:### Thermodynamic Tables
These tables provide essential thermodynamic data for various substances, including their standard enthalpy of formation (ΔHf°) and standard free energy of formation (ΔGf°), along with atomization and bond energies.
#### Standard Enthalpy of Formation (ΔHf°) Values
| Substance | kJ/mole |
|-------------|----------|
| CO(g) | -110 |
| CO₂(g) | -394 |
| CH₃OH(l) | -238 |
| CH₄(g) | -75 |
| C₂H₄(g) | +52 |
| Fe₂O₃(s) | -824 |
| H₂O(l) | -286 |
| H₂O(g) | -242 |
| NaCl(s) | -413 |
| NaOH(aq) | -470 |
| NH₃(g) | -46 |
| NO(g) | +90 |
| C₂H₅OH(l) | -485 |
| HC₂H₃O₂(l) | -278 |
#### Standard Free Energy of Formation (ΔGf°) Values
| Substance | kJ/mole |
|-------------|----------|
| CO(g) | -137 |
| CO₂(g) | -395 |
| Fe₂O₃(s) | -742 |
| H₂O(l) | -237 |
| H₂O(g) | -228 |
| NH₃(g) | -17 |
| NO(g) | +87 |
| NO₂(g) | +51 |
| N₂O₄(g) | +98 |
| C₂H₅OH(l) | -390 |
| HC₂H₃O₂(l) | -175 |
#### Atomization Energies
| Atom | kJ/mole atom |
|-------|--------------|
| C(g) | 715 |
| Cl(g) | 121 |
| H(g) | 218 |
| I(g) | 107 |
| Na(g) | 108 |
| O(g) | 249 |
|
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Please don't provide handwritten solution ....arrow_forwardwww-awn.aleks.com/alekscgi/x/Isl.exe/1o_u- Islkr/18P3]H-lvUg4xjUlp4GtU74E36QsaO3tOVD_LL5vyaAlqw-Z0OQCDKEYTYH O THERMOCHEMISTRY Interconverting calories and joules Complete the following table. Be sure each of your answer entries has the correct number of significant digits. energy content when eaten food cal kcal kJ a small apple 1.25 × 10 a tablespoon of corn oil 14.0 a cup of grapefruit juice 481. IIIarrow_forward[Review Topics] [References] Use the References to access important values if needed for this question. For the reaction 2BrF3(g) Br2(g) + 3F2(g) AH° = 542 kJ and AS° = 269 J/K AG° would be negative at temperatures (above, below) K. Enter above or below in the first box and enter the temperature in the second box. Assume that AH° and AS° are constant. Submit Answer Retry Entire Group 4 more group attempts remaining edarrow_forward
- A east.cengagenow.com 山 OWLV2 | Online teaching and learning resource from Cengage Le... [Review Topics] [References] Use the References to access important values if needed for this questlon. For the reaction H2(g) + C,H,(g) C,H,(g) AG° =-103.0 kJ and AS° = -120.7 J/K at 282 K and 1 atm. The maximum amount of work that could be done by this reaction when 2.35 moles of H,(g) react at standard conditions at this temperature is kJ. Submit Answer Retry Entire Group 1 more group attempt remaining In progress Next jiarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY