
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
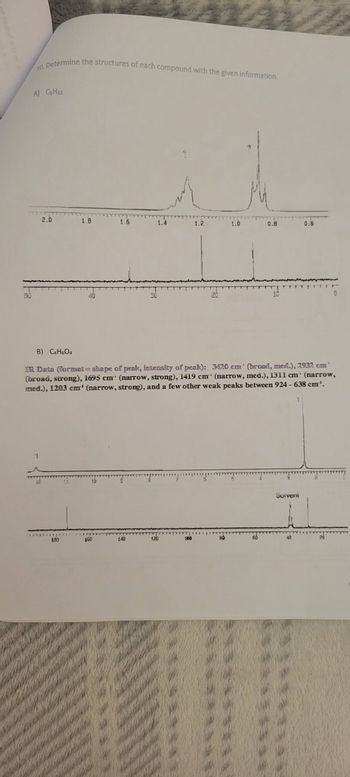
Transcribed Image Text:10. Determine the structures of each compound with the given information.
A) CsH12
2.0
1.2
1.8
LEO
40
1.5
160
30
140
1.4
1.20
1.2
100
20
B) C4H6O4
IR Data (format= shape of peak, intensity of peak): 3420 cm' (broad, med.), 2932 cm
(broad, strong), 1695 cm (narrow, strong), 1419 cm¹ (narrow, med.), 1311 cm (narrow,
med.), 1203 cm' (narrow, strong), and a few other weak peaks between 924- 638 cm¹.
1.0
BO
Mu
0.8
10
1
0.5
Soivent
0
www.qq.cong
20
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help with Org chem pleasearrow_forward1. For the following compounds, determine the following: A. Number of peaks, B. Integral of each peak, C. Estimate of each peak location (ppm), D. Splitting pattern for each peak (singlet, doublet, triplet, etc.) For example, ethanol (CH3CH2OH) would be 3 peaks: CH3 peak at -1.5 ppm (3H, triplet), CH2 at -3.5 ppm (2H, quartet), and OH peak at -4.5 ppm (1H, singlet). This is dihydropyran, which is used as a "protecting group" for alcohols during organic synthesis. Protecting groups are chemical modifications that mask a functional group and prevent an undesired side reaction from occurring. This is a common reagent called "Boc anhydride" (like the sound a chicken makes - boc boc boc) and is used as a protecting group for amines during peptide synthesis. HO ΟΗ OH СОН This is the meso form of tartaric acid. Because it's a meso compound, this affects the number of peaks in its NMR spectrum.arrow_forwardPlease identify the compound present in the IR and MS scans. Both scans utilized the same compound. (Each graph is labled with IR and MS respectively)arrow_forward
- Select the structure that best fits the IR data shown. The correct molecular formula Which of these molecules best corresponds to the IR spectrum below? i 0.8 should be C5H120. 0.6 0.4 0.2 3000 b) C₂H₂O c) 2000 H Wavenumber (cm-1) COPE 1000 d) OHarrow_forwardGiven the mass spectrum above, what is the most likely molecular formula for this molecule? OCH₂CIBr more data needed ●CH₂Cl₂ OCH₂Br₂arrow_forward15. Draw the structure for the compound at the bottom of the page. Show your reasoning IR Spectrum (lquld fim) 1715 4000 3000 2000 1600 1200 0,0 800 V (om) 100 43 Mass Spectrum 80 0.6 60 UV spactrum 40 1.0 Mt 134 Balvent : cyclohexane 7.00 mg/10 mls palh langth:0.5o am 20 CgH100 1.5 40 80 120 160 m/e 200 240 280 200 250 300 350 2(nm) 13C NMR Spectrum (50.0 MHz, CDCI, solution) DEPT CHat CH,t CHt prolon decoupled aclvent 200 160 120 80 40 8 (ppm) H NMR Spectrum (200 MHz, CDCI, colution) 5H 2H 3H TMS 10 8 4 2 *Spectra from "Organic Structures from Spectra", Field, Sternbell and Kalman 0. 8 (ppm) % of base peak absorbancearrow_forward
- Propose a structure that is consistent with the following MS spectral data. m/z Intensity 121(M*) 47.9 122 4.0 Fill in the box next to each of the elements (C, H, N, O) with the number of atoms for the element that would appear in the proposed formula. Fill in "O" if there is no such element in the formula. Do not leave any boxes blank. Harrow_forward8.Label the functional groups and identify the correct compound based on the IR spectrum. CI Br % Transmittance a) 100 50 0 4000 b) OH gorda d) 3000 CI CI CI CI CI c) mm 2000 1500 Wavenumber (cm-¹) Chemistry T 1000 500arrow_forwardWhat is the structure of the compound whose mass spectrum is depicted below? Relative Intensity A) 100 80 60 40 20 10 tuttin 20 COOH 40 B) 60 80 4444 C) 100 120 Br D) 140 160 CI 180 200arrow_forward
- I I I A molecule produces an IR spectrum with key peaks at approximately 3300 and 2100 cm¯¹. The mass spectrum has a molecular ion with a m/z = 68, and major fragment with m/z of 39. Draw a structure that best fits this data. Drawing I Iarrow_forwardPredict the relative intensities of the M and M+ 2 peaks for the following. Q) CH3CH2SHarrow_forwardCan you please help with the organic chemistry question attached?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY