
Principles of Physics: A Calculus-Based Text
5th Edition
ISBN: 9781133104261
Author: Raymond A. Serway, John W. Jewett
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
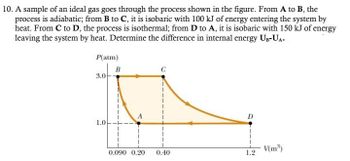
Transcribed Image Text:10. A sample of an ideal gas goes through the process shown in the figure. From A to B, the
process is adiabatic; from B to C, it is isobaric with 100 kJ of energy entering the system by
heat. From C to D, the process is isothermal; from D to A, it is isobaric with 150 kJ of energy
leaving the system by heat. Determine the difference in internal energy UB-UA.
P(atm)
B
3.0
1.0
0.090 0.20
0.40
1.2
V(m³)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (a) How much food energy will a man metabolize in the process of doing 35.0 kJ of work with an efficiency of 5.00%? (b) How much heal transfer occurs to the environment to keep his temperature constant? Explicitly show how you follow the steps in the Problem—Solving Strategy for thermodynamics found in Problem-Solving Strategies for Thermodynamics.arrow_forwardThere is no change in the internal of an ideal gas undergoing an isothermal process since the internal energy depends only on the temperature. Is it therefore correct to say that an isothermal process is the same as an adiabatic process for an ideal gas? Explain your answer. `arrow_forwardThis problem compares the energy output and heat transfer to the environment by two different types of nuclear power stationsone with the normal efficiency of 34.0%, and another with an improved efficiency of 40.0%. Suppose both have the same heat transfer into the engine in one day. 2.501014J. (a) How much more electrical energy is produced by the more efficient power station? (b) How much less heat transfer occurs to the environment by the more efficient power station? (One type of more ef?cient nuclear power station, the gas—cooled reactor, has not been reliable enough to be economically feasible in spite of its greater eficiency.)arrow_forward
- Suppose an ideal (Carnot) heat pump could be constructed for use as an air conditioner. (a) Obtain an expression for the coefficient of performance (COP) for such an air conditioner in terms of Tb and Tc. (b) Would such an air conditioner operate on a smaller energy input if the difference in the operating temperatures were greater or smaller? (c) Compute the COP for such an air conditioner if the indoor temperature is 20.0C and the outdoor temperature is 40.0C.arrow_forward(a) How much heat transfer occurs from 20.0 kg of 90.0C water placed in contact with 20.0 kg of 10.0C water, producing a final temperature of 50.0C ? (b) How much work could a Carnot engine do with this heat transfer, assuming it operates between two reservoirs at constant temperatures of 90.0C and 10.0C ? (c) What increase in entropy is produced by mixing 20.0 kg of 90.0C water with 20.0 kg of 10.0C water? (d) Calculate the amount of work made unavailable by this mixing using a low temperature of 10.0C, and compare it with the work done by the Garnet engine. Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy. (e) Discuss how everyday processes make increasingly more energy unavailable to do work, as implied by this problem.arrow_forwardYou are working on a summer job at a company that designs non-traditional energy systems. The company is working on a proposed electric power plant that would make use of the temperature gradient in the ocean. The system includes a heat engine that would operate between 20.0C (surface-water temperature) and 5.00C (water temperature at a depth of about 1 km). (a) Your supervisor asks you to determine the maximum efficiency of such a system. (b) In addition, if the electric power output of the plant is 75.0 MW and it operates at the maximum theoretically possible efficiency, you must determine the rate at which energy is taken in from the warm reservoir. (c) From this information, if an electric bill for a typical home shows a use of 950 kWh per month, your supervisor wants to know how many homes can be provided with power from this energy system operating at its maximum efficiency. (d) As energy is drawn from the warm surface water to operate the engine, it is replaced by energy absorbed from sunlight on the surface. If the average intensity absorbed from sunlight is 650 W/m2 for 12 daylight hours on a clear day, you need to find the area of the ocean surface that is necessary for sunlight to replace the energy absorbed into the engine. (e) From this information, you need to determine if there is enough ocean surface on the Earth to use such engines to supply the electrical needs for all the homes associated with the Earths population. Assume the energy use for a home in part (c) is an average over the entire planet. (f) In view of your results in this problem, your supervisor has asked for your conclusion as to whether such a system is worthwhile to pursue. Note that the fuel (sunlight) is free.arrow_forward
- (a) In reaching equilibrium, how much heat transfer occurs from 1.00 kg of water at 40.0C when it is placed in contact with 1.00 kg of 20.0C water in reaching equilibrium? (b) What is the change in entropy due to this heat transfer? (c) How much work is made unavailable, taking the lowest temperature to be 20.0C ? Explicitly show how you follow the steps in the Problem-Solving Strategies for Entropy.arrow_forwardAn ideal gas is compressed to half its initial volume by means of several possible processes. Which of the following processes results in the most work done on the gas? (a) isothermal (b) adiabatic (c) isobaric (d) The work done is independent of the process.arrow_forwardUse a PV diagram such as the one in Figure 22.2 (page 653) to figure out how you could modify an engine to increase the work done.arrow_forward
- A coalfired electrical power station has an efficiency of 38%. The temperature of the steam leaving the boiler is 550°C. What percentage of the maximum efficiency does this station obtain? (Assume the temperature of the environment is 20C .)arrow_forward(a) On a winter day, a certain house loses 5.00108J of heat to the outside (about 500,000 Btu). What is the total change in entropy due to this heat transfer alone, assuming an average indoor temperature of 21.0C and an average outdoor temperature of 5.00C ? (b) This large change in entropy implies a large amount of energy has become unavailable to do work. Where do we find more energy when such energy is lost to us?arrow_forward(a) What is the eficiency of a cyclical heat engine in which 75.0 kJ of heat transfer occurs to the environment for every 95.0 kJ of heat transfer into the engine? (b) How much work does it produce for 100 kJ of heat transfer into the engine?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
 Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781938168000
Author:Paul Peter Urone, Roger Hinrichs
Publisher:OpenStax College


Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning