
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
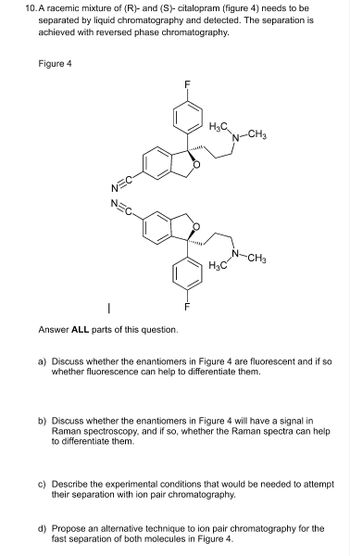
Transcribed Image Text:10. A racemic mixture of (R)- and (S)- citalopram (figure 4) needs to be
separated by liquid chromatography and detected. The separation is
achieved with reversed phase chromatography.
Figure 4
F
F
H3C
N-CH3
-CH3
H3C
Answer ALL parts of this question.
a) Discuss whether the enantiomers in Figure 4 are fluorescent and if so
whether fluorescence can help to differentiate them.
b) Discuss whether the enantiomers in Figure 4 will have a signal in
Raman spectroscopy, and if so, whether the Raman spectra can help
to differentiate them.
c) Describe the experimental conditions that would be needed to attempt
their separation with ion pair chromatography.
d) Propose an alternative technique to ion pair chromatography for the
fast separation of both molecules in Figure 4.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- In an experiment of Reverse Phase chromatography using C-18 stationary phase and a mixture of methanol/ethyl acetate/acetic acid, a researcher tried to separate the 3 compounds shown below. Which compound would have the lowest Rf and which the highest Rf in this experiment? Explain your answer and show any relevant structures.arrow_forwardWhy is distillation of cyclohexanol using acid catalyst phosphoric acid a better technique to purofy rather than extraction, recrystallization, chromatography, IR spectroscopy?arrow_forwardPayalbenarrow_forward
- Z and E -oct-3-en-2-ol is hard or easy to separate in thin layers in chromatography?arrow_forwarda. Say you are trying to separate two compounds by column chromatography. You run a column and collect the eluent in five fractions. You analyze each fraction by TLC on the same plate (shown below). Based on the TLC plate, how effective was your separation? Consider the purity of each fraction. - - - - - - - - ----| 1 2 3 4 5arrow_forwardWhat would happen to the retention time of a compound if the following changes were made? Sketch the chromatogram of this mixture for the following questions (a, b and c). "10א2s 2.5 1.5- 0.5 -05 4.2 4.6 5.2 54 5.6 5.8 Time (s) a. Increase the flow rate of the carrier gas b. Decrease the temperature of the column c. Decrease the length of the column Intensity (arb, units)arrow_forward
- The following chromatographic peaks indicate the undesirable interaction and separation that taken place. Explain in detail how these undesirable interaction and separation happen for each chromatographic peak. b с d aarrow_forwardIn the diagram of TLC for column chromatography, What does light faded looking spots (less or less product, etc.)? Which fractions contain the product? concentrated 1 2 3 4 14 26 15 27 16 28 17 29 5 6 7 8 18 19 30 31 32 20 33 9 21 10 22 11 23 12 13 24 25 34 35 36 37 38arrow_forwardWhich one is the more polar component? Explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY