
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
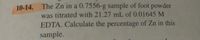
Transcribed Image Text:10-14. The Zn in a 0.7556-g sample of foot powder
was titrated with 21.27 mL of 0.01645 M
EDTA. Calculate the percentage of Zn in this
sample.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer 8darrow_forwardA 100.0 mL, 0.953 M acetic acid solution was titrated with 121.4 mL of 0.785 M NaOH. Find the pH of the solution. The Ka of acetic acid is 1.8 x 10-5arrow_forward17- Consider the following titration curve that corresponds to the titration of a weak acid with a strong base. From the data shown, what is the approximate Ka of the acid?arrow_forward
- > C V B M Alt Alt Ctrl + Hc 9. I.C. Calculate the volume of a 0.50 M NaOH(aq) necessary to completely neutralize 50.0 mL of an aqueous 0.1 M CH3COOH solution. This would be considered equimolar amounts of acid and base (a.k.a. the equivalence point of a titration). Show your work.arrow_forwardIn the potentiometric titration of phosphoric acid, pH readings at the first and second half-equivalence points were pH1 = 2.12 and pH2 = 7.21 respectively. Determine the Ka1 and Ka2 for phosphoric acid, rounded to 3 sig figs. At the half-equivalence points, pH = pKa. Also, pH = –log [H+] and pKa = –log Ka. Type your answers one below the other. Use formatting tools where applicable.arrow_forwardAnswer 8Farrow_forward
- 10-6. Consider the titration of 100.0 mL of 0.100 M NaOH with 1.00 M HBr. What is the equivalence volume? Find the pH at the following volumes of HBr and make a graph of pH versus Va: Va = 0, 1.00, 5.00, 9.00, 9.90, 10.00, 10.10, and 12.00 mL.arrow_forward1. What is the pH at the half-stoichiometric point (a.k.a. mid-point) for the titration of 0.22 M acetic acid(aq) with 0.10 M KOH(aq)? For acetic acid, Ka = 1.8 × 10-5.arrow_forward3:21 1 4 7 +/- Question 7 of 10 In the titration of 230.0 mL of 0.4000 M HONH₂ with 0.2000 M HBr, how many mL of HBr are required to reach the equivalence point? 2 5 8 mL 3 60 9 O Submit Tap here or pull up for additional resources XU x 100arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY