
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please answer fast i give upvote
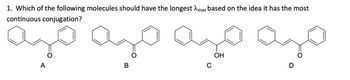
Transcribed Image Text:1. Which of the following molecules should have the longest max based on the idea it has the most
continuous conjugation?
ಂ
ОН
C
A
B
D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Which of the following molecules should have the longest max based on the idea it has the most continuous conjugation? A B C OH Darrow_forwardWhich of these molecules are polar? For each that is polar, specify the direction of its dipole moment. Q) CH3Clarrow_forwardClick on all of the atoms that make up the largest coplanar unit in the molecule below. XX H H -Harrow_forward
- Which molecule would be more polar, CH3F or CH3Cl? And why?arrow_forwardYou will need to draw the chair forms (and possibly the flipped chair forms) of the molecules in each row. (HINT: "most stable" is most big groups equatorial.) Most stable in row 1 is Most stable in row 2 Most stable in row 3arrow_forwardWhich of the highlighted chemical bonds in the molecules below is longest? Shortest? In between? Which highlighted bond requires the highest energy to break? Lowest? In between? Answer these questions by completing the second and third columns in the table. compound H-C=N-H I H H H | | H-C-N-H I H H-CEN: length of highlighted bond choose one - choose one - choose one energy of highlighted bond choose one - choose one - V - choose one X 3arrow_forward
- Match each of the general molecular formulas below with its correct molecular geometry. Note: The sorting box Tri. Bipyramidal stands for trigonal bipyramidal. Items (6 items) (Drag and drop into the appropriate area below) AB5 with no lone pairs on A AB4 with one lone pair on A AB4 with two lone pairs on A AB2 with one lone pair on A AB2 with two AB2 with no lone lone pairs on A pairs on A Categories Bent 120° bond Bent 109.5° bond Tri. bipyramidal Square planar Seesawarrow_forwardMake a continuous model for C4H10 by using 4 black 4 hole carbon atoms, 10 white one hole hydrogen atoms, and 13 pink bonds. Then write in wedge dash notation C4H10 and add in missing hydrogen atoms.arrow_forward3. Which of the following molecules are conjugated? For those which are conjugated, write a resonance structure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning