
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
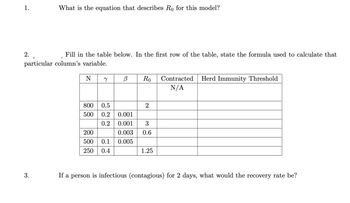
Transcribed Image Text:1.
What is the equation that describes Ro for this model?
2..
Fill in the table below. In the first row of the table, state the formula used to calculate that
particular column's variable.
N y
3.
B
800 0.5
500 0.2 0.001
0.2 0.001
200
0.003
500 0.1 0.005
250 0.4
Ro
2
3
0.6
1.25
Contracted Herd Immunity Threshold
N/A
If a person is infectious (contagious) for 2 days, what would the recovery rate be?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- 5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 a DCM 23 Tor • DCM 50 Torr x DCM 100 Torr Symbols: o, 0 Torr DCM; O, 23 Torr DCM A, 50 Torr DCM x, 100 Torr DCM. 80 40 (1 Torr = 1 mm Hg.) The solutions buffered to pH 7.4. 20 were 0.5 1.0 1.5 2.0 log po2 % OXYGENATIONarrow_forward4 How do I convert from DPM to picomoles per milligram of protein (pmol/mg)? I have the specific activity as [3H]-spiperone is 25530 DPM/pmol. is there an equation?arrow_forward25. Assume we are performing a 2x serial dilution series of methylene similar to WL-1. If your original, 1x tube is designated "Tube 1", then what would be the relative concentration of methylene blue in Tube 4? a. Tube 4 is the 8x dilution tube. The concentration of methylene blue in this tube is 1/8 that of the original tube. b. Tube 4 is the 8x dilution tube. The concentration of methylene blue in this tube is 8 times that of the original tube. c. Tube 4 is the 4x dilution tube. The concentration of methylene blue in this tube is 4 times that of the original tube. d. Tube 4 is the dilution 10x tube. The concentration of methylene blue in this tube is 1/10 that of the original tube. e. None of the abovearrow_forward
- 3. Consider the molecule dopamine (shown). Given that its pKa(NH3¹) = 10.9. a. What pH range would dopamine be a good buffer at? b. Draw the structures of the predominant species in solution at (i) pH 7, (ii) pH 10, and (iii) pH 12. c. At what pH range do you expect dopamine to be most water soluble? d. At what pH do you expect dopamine to be most hexane soluble? НО. HO + NH3 pka = 10.9arrow_forward7. [10'] For a bacteriophage 77, the following data at zero concentration have been obtained: Sedimentation coefficient: $20,w = 453 S. Diffusion coefficient: D₂0. = 6.03×10-8 cm² s¹¹. 20,w Specific volume V20 = 0.639 cm³ g¹ Calculate the molecular mass M of the bacteriophage.arrow_forward3. Consider a hypothetical chemical reaction: WKNO3 → xK + yN2 +zO2. (a) Write a system of 3 equations in the 4 variables w, x, y, z that represents the reaction. (b) Explain why the only solution is w = x = y = z = 0. That is, the reaction is impossible for mathematical reasons.arrow_forward
- 5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 a Dсм 23 Torr Symbols: в Dсм 50 Torr x DCM I00 Torr o, 0 Torr DCM; O, 23 Torr DCM A, 50 Torr DCM x, 100 Torr DCM. 80 60 40 (1 Torr = 1 mm Hg.) 20 The solutions were buffered to pH 7.4. 0.5 1.0 1.5 2.0 log p02 (a) (* Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the value of the dissociation equilibrium constant Ka for O2 binding is defined for 0 and 100 Torr of dichloromethane For the curves at 0 and 100 Torr of dichloromethane, draw their equivalent in the form of Kd Hb(O2)n Hb + nO2 % OXYGENATIONarrow_forwardHow would I create a bar graph using the Hardy -Weinberg Equation data ?arrow_forward) In the thin layer chromatography of practical 5 the chromatography tank contained a mixture of n-butanol:acetic acid:water (2:1:1) as the developing solvent. i) predict what would happen to the migration of the sugars if you would use butane:acetic acid:water (10:1:1) as the developing solvent; and ii) provide a rationale for your prediction that takes into account the principle of TLC separation, and the chemical properties of the sugars. The chemical spot tests for carbohydrates and reducing sugars on thesesamples as outlined below: 1.Glucose2. Sucrose3. Fructose4. Unknown5. Lactose6. Galactose7. Maltose8. Honey9. Ripe banana10. Starch11. Distilled water (control)arrow_forward
- 22. For the process, A B, Keq is 0.02 at 37°C. For the process, BC, Keq 1000 at 37°C. A. Determine Keq (AC), the equilibrium constant for the overall process A C, from Keq (AB) and Keq (BC). B. Determine standard-state free energy changes for all three processes and use AG'(AC) to determine Keq (AC). Make sure that this value agrees with that determined in part A of this problem.arrow_forward5.Myasthenia (muscle weakness)is characterized by damage in the transmission of neurotic impuise and decreased amount of acetylcholine due to autoimmune mechanisms. Prozerine is used for treatment of this disease.Specify the mechanism of this drug action. For this: 1)Write the reaction of acetylcholine degradation at neuromuscular junction. 2)What enzyme will be inhibited with prozerine? Name the class of this enzyme. 3)What is the mechanism of this enzyme inhibition?arrow_forward1. Start with the Michaelis-Menten equation and convert it to a double-reciprocal equation. Show how you would make a linear Lineweaver-Burk plot using this equation. 2. Based on th chart make a Lineweaver-Burk plot and later use the plot to complete the information in the table.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education