
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
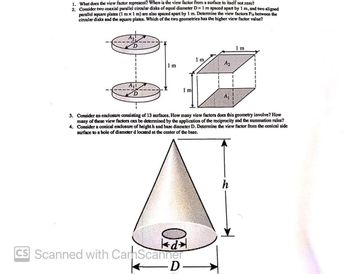
Transcribed Image Text:1. What does the view factor represent? When is the view factor from a surface to itself not zero?
2. Consider two coaxial parallel circular disks of equal diameter D = 1 m spaced apart by 1 m, and two aligned
parallel square plates (1 mx 1 m) are also spaced apart by 1 m. Determine the view factors F₁2 between the
circular disks and the square plates. Which of the two geometries has the higher view factor value?
D
D
1 m
1 m
kd
CS Scanned with CamScanner
1 m,
D-
A2
3. Consider an enclosure consisting of 13 surfaces. How many view factors does this geometry involve? How
many of these view factors can be determined by the application of the reciprocity and the summation rules?
4. Consider a conical enclosure of height h and base diameter D. Determine the view factor from the conical side
surface to a hole of diameter d located at the center of the base.
Im
h
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- Consider an enclosure consisting of a hemisphere of diameter D and a flat surface of the same diameter Determine the relevant view factors. D/2arrow_forwardhi, please draw a simple drawing that is easy to drawarrow_forwardNormalize the wavefunction that is valued at Cx1/2 between x = 0 and x = L, and is 0 everywhere outside this box of length L.arrow_forward
- For a hypothetical crystal structure, the {212} family of planes and <110> family of directions have the highest planar and linear densities, respectively. Determine all possible slip systems of the crystal structure.arrow_forward2C.4 Falling-cylinder viscometer (see Fig. 2C.4). A falling-cylinder viscometer consists of a long vertical cylindrical container (radius R), capped at both ends, with a solid cylindrical slug (ra- dius KR). The slug is equipped with fins so that its axis is coincident with that of the tube. One can observe the rate of descent of the slug in the cylindrical container when the lat- ter is filled with fluid. Find an equation that gives the viscosity of the fluid in terms of the ter- minal velocity of the slug and the various geometric quantities shown in the figure. Cylindrical slug descends- with speed t -XR- Cylindrical container filled with fluid Fig. 2C.4 A falling-cylinder viscom- eter with a tightly fitting solid cylin- der moving vertically. The cylinder is usually equipped with fins to maintain centering within the tube. The fluid completely fills the tube, and the top and bottom are closed. (a) Show that the velocity distribution in the annular slit is given by (1-)-(1+¹) In…arrow_forwardProblem 3: A model equation for chemical reactive flow in a one-dimensional reactor is given as ac ac +u. ôt dx :DOC-KC, where C is the dimensionless concentration of the species, u is the velocity, D is a diffusion coefficient, k is a reaction rate, and x,t are the distance from the reactor and time, respectively. (3a) Determine the dimensions of D, k;arrow_forward
- the question to be answered in 2 nd imagearrow_forwardAt 293 K Benzene flows through viscometer 1.8 times slower than acetone. What is the density ratio of acetone to benzene based on viscosity? Assume that viscosity of acetone at 293 K is the same as 298 K.arrow_forwardplz answer both 2 questionsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The