
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
the question to be answered in 2 nd image
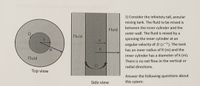
Transcribed Image Text:1) Consider the infinitely tall, annular mixing tank. The fluid to be mixed is between the inner cylinder and the outer wall. The fluid is mixed by a spinning the inner cylinder at an angular velocity of Ω (s⁻¹). The tank has an inner radius of R (m) and the inner cylinder has a diameter of k (m). There is no net flow in the vertical or radial directions.
Answer the following questions about this system:
**Diagrams Explanation:**
- **Top View Diagram:**
- Displays a circular tank with an inner and outer part.
- The inner circle represents the rotating cylinder with an angular velocity of Ω, and its radius is indicated by "k".
- The space between the inner cylinder and the outer boundary of the tank holds the fluid to be mixed. The outer radius of the tank is labeled "R".
- **Side View Diagram:**
- Shows a vertical cross-section of the tank.
- The inner cylinder is rotating with angular velocity Ω.
- The fluid is present in the annular space between the rotating inner cylinder and the stationary outer wall.
- The parameters k and R are also illustrated for clarity.
These diagrams serve to visualize the setup of the mixing tank from both the top and side perspectives, explaining how the fluid is mixed in the annular region.

Transcribed Image Text:2. Simplify the r-equation of motion (remove zero terms) and write it below:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Final answer in three decimals. Show the solution.arrow_forwardU0zden GEÇir Görünüm Aa- E-= - EE T Aralık Yok T Konu Ba. T Liste Par.. T Normal Paragraf Stiller Derive the transfer function of the process from the block diagram shown in the figure. G,(s) X(s)} G (s) Y(s) G (s) G(s) G(5). SONY REDMI NOTE 8 AI QUAD CAMERAarrow_forwardFluid Mechanics easy questionarrow_forward
- 1) Pavithran Ravindran explains to a group of inspiring CBE students that the Reynolds number is a dimensionless group defined for a fluid flowing in a pipe as Re = Dvp/µ where D = pipe diameter, v = average fluid velocity, p = fluid density, and u = fluid viscosity. When the value of the Reynolds number is less than about 2100, the flow is laminar -- the fluid flows in smooth streamlines. For Reynolds numbers above 2100, flow is turbulent -- a great deal of internal agitation takes place. %3D Methyl ethyl ketone (MEK) flows through a 2.067-in. ID pipe at 20°C, at which temperature its density is 0.805 g/cm³ and its viscosity is 0.43 centipoise (1 centipoise = 1.00 x 10-3 kg/m.s). The average fluid velocity is 0.048 ft/s. Determine whether the flow is laminar or turbulent. %3Darrow_forwardchemical engineering A power plant emits 216 g of SO2 every hour. The wind recorded on the same day is 5 m s-1, and the atmospheric stability class is A for the clear summer afternoon. There is no plume rise, and the stack height is 30 m. The values of σy and σz are 215 m and 450 m, respectively. Determine: the concentration of SO2 0.7 km downwind along the plume centreline at ground level.arrow_forward1. Cold water is flowing into and get heated in a pipe wrapped with electrical heating wires. At a certain heating power P, the temperature profiles is given on the left below. If the cold water temperature (Tm,in) and mass flow rate remains the same, but the electrical heater power increases, how would the temperature profiles look like? Sketch on the right below and provide brief explanation (less than 100 words). TS Tm,out Tm,in Tm Tm,inarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The