
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
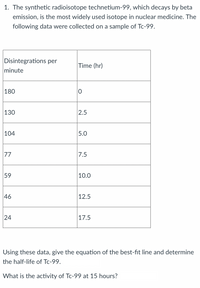
Transcribed Image Text:1. The synthetic radioisotope technetium-99, which decays by beta
emission, is the most widely used isotope in nuclear medicine. The
following data were collected on a sample of Tc-99.
Disintegrations per
Time (hr)
minute
|180
130
2.5
104
5.0
77
7.5
59
|10.0
46
12.5
|17.5
Using these data, give the equation of the best-fit line and determine
the half-life of Tc-99.
What is the activity of Tc-99 at 15 hours?
24
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 18.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. sample A B C D symbol 179 73 91 39 103 46 59 radionuclide 26 Ta Y Pd Fe half-life 2.0 years 59.0 days 17. days 45.0 days initial radioactivity (choose one) (choose one) ✓ (choose one) ✓ (choose one) ✓ time for amount of radionuclide to decrease to 1/32 of initial amount years days days daysarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 22.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/8 of the initial amount. sample A B C D symbol 75 34 133 54 95 41 227 radionuclide 89 Se Xe Nb Ac half-life 120. days 5.0 days 35.0 days 22. years initial radioactivity (choose one) î (choose one) ↑ (choose one) ŵ (choose one) ↑ time for amount of radionuclide to decrease to 1/8 of initial amount X days days days years Śarrow_forwardRadioactive decay can be described by the following equation In A = In Ao – kt where Ao is the original amount of the substance, A is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. -1 For the radioactive isotope lead-210, k is 3.30 x 10 years If the original amount of lead-210 in a sample is 60.0 mg, how much lead-210 remains after 24.6 years have passed? mgarrow_forward
- This experiment will use a technique known as neutron activation analysis (NAA) to determine the half-lives of two unusual isotopes, 28 Al and 20F. The only stable isotopes of these elements are 27 Al and 19F. Trace amounts of 26 Al and 18F occur naturally, but the isotopes we will generate today are even more unstable and have no significant natural abundance. To produce these very unstable isotopes in measurable concentration, samples containing Al and F are placed next to a source of energetic neutrons. A number of neutron sources can be used; in this lab the samples are placed inside an operating nuclear reactor that emits energetic neutrons. This source has the advantage of a high neutron output that creates the desired isotopes rapidly and at higher concentrations, but it is only feasible for researchers that have access to nuclear reactors. At Oregon State University, the campus TRIGA reactor is set up for NAA experiments. SECTION 2 Based on the description from the last…arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 8.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. sample A B C D symbol 82 37 127 54 149 65 91 radionuclide 39 Rb Xe Tb Y half-life 1. minute 36.0 days 4.0 hours 59.0 days initial radioactivity (choose one) (choose one) ✓ (choose one) ✓ (choose one) ✓ time for amount of radionuclide to decrease to 1/16 of initial amount minutes days hours daysarrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 μmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. sample A B D symbol 18 9 75 M 35 177 71 152 63 radionuclide F Br Lu Eu half-life 2. hours 98.0 minutes 7.0 days 13. years initial radioactivity (choose one) (choose one) O (choose one) (choose one) time for amount of radionuclide to decrease to 1/16 of initial amount hours minutes days yearsarrow_forward
- Cancer therapy with radioactive rhenium-188 shows promise in patients suffering from colorectal cancer. a. Write the symbol for rhenium-188 and determine the number of neutrons, protons, and electrons. b. The half-life of rhenium-188 is 17 hours. If it takes 30 minutes to bind the isotope to an antibodythat delivers the rhenium to the tumor, what percentage of the rhenium remains after binding tothe antibody?arrow_forwardIdentiy the missing particle in the following nuclear equation. Please help! How do i DO THIS?arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 13.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. radionuclide sample initial radioactivity symbol half-life 46 A Sc 84.0 days (choose one) 21 21 time for amount of radionuclide to decrease to 1/16 of initial amount ☐ days 91 B Y 59.0 days (choose one) ☐ days 39 ur 52 C Mn 6.0 days (choose one) ☐ days 25 18 D F 2. hours (choose one) hours 9 1 (highest) half 2 3 4 (lowest) х Ar ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY