
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
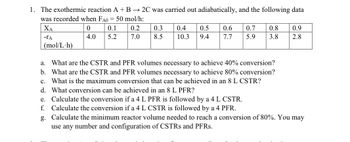
Transcribed Image Text:1. The exothermic reaction A + B 2C was carried out adiabatically, and the following data
was recorded when FA0 = 50 mol/h:
2
ΧΑ
0
0.1
-ΤΑ
4.0
5.2
(mol/L.h)
67
0.2
0.3 0.4
0.5
0.6
7.0
8.5
10.3 9.4 7.7
0.7
05
0.8
03
79
5.9
88
3.8
0.9
2.8
a. What are the CSTR and PFR volumes necessary to achieve 40% conversion?
b. What are the CSTR and PFR volumes necessary to achieve 80% conversion?
c. What is the maximum conversion that can be achieved in an 8 L CSTR?
d. What conversion can be achieved in an 8 L PFR?
e. Calculate the conversion if a 4 L PFR is followed by a 4 L CSTR.
f. Calculate the conversion if a 4 L CSTR is followed by a 4 PFR.
g. Calculate the minimum reactor volume needed to reach a conversion of 80%. You may
use any number and configuration of CSTRS and PFRs.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 9 images

Knowledge Booster
Similar questions
- QUESTION 2 Regarding the following reaction at 298.15 K: 4 HCI (g) + O2 (g) →→ 2Cl₂ (g) + 2H₂O (1) The following table lists the stand enthalpy of formation, the standard entropy and the standard formation Gibbs energies Cl2 (g) O2 (g) 0 H₂O (1) -285.83 0 HCI (g) -92.31 186.91 -95.3 69.91 4+Ho(kJmol-1) sº (JK-1mol-1) 4Gº (kJmol-1) 205.14 0 223.07 0 ? Please calculate the standard reaction entropy 4,S°(JK-1mol-1), the standard reaction enthalpy Hº(kJmol-1), the standard reaction Gibbs energy 4,Gº(kJmol-1), and the maximum nonexpansion work that can be gained from the oxidation of HCI (g) at 298.15K. Instruction: Please enter all answers with two decimal places, for example: -344.101 is written as -344.10, please pay attention to the units, particularly As in J/(K.mol) while others is kJ/mol (1) 4,5°(JK-1 mol-1) = (2) 4,H°(kJmol-1) = Please using your results from (1) and (2) to calculate the the standard reaction Gibbs energy:arrow_forward2. Consider the following reaction at constant P. Determine the value of ASsurr at 298 K, then predict whether this reaction will be spontaneous at this temperature. N2(g) + 2 02(g) → 2 NO2(g) AH = +66.4 kJarrow_forwardWhat is the value of the equilibrium constant at 298 K for a reaction that has a ΔGo of +64.29 kJ/mol?arrow_forward
- 13.86. The rate constant for the reaction NO₂(g) + O3(g) → NO3(g) + O₂(g) was determined over a temperature range of 40 K, with the following results: T (K) 203 213 223 233 243 k (M¹s¹) 4.14 X 105 7.30 X 105 1.22 X 106 1.96 X 106 3.02 X 106 a. Determine the activation energy for the reaction. b. Calculate the rate constant of the reaction at 300 K.arrow_forwardConsider the following reaction where K. = 1.29×102 at 600 K. COCI2(g) CO(g) + Cl2(g) A reaction mixture was found to contain 0.109 moles of COC1,(g), 2.19x10 2 moles of CO(g), and 3.88x102 moles of Cl,(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qe, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forward4) All right... hypofluorite! At 294 K, the gas phase reaction CF3 OF + C3 F6 → C3F7OCF3 has been found to follow this Rate Law: Rate = k [CF3 OF]3/2 [C3F6]!/2 If Ea = 52.0 kJ mol-1, and the temperature drops by 8 K, by what multiplier would you change the initial concentration of hypofluorite (only) so that the initial rate is unchanged? My concentration will be: larger - by a factor of: 0.125 Evaluate Extra Infoarrow_forward
- Consider the following system at equilibrium where AH° = -198 kJ/mol, and K. = 34.5 , at 1150 K. 2 SO2 (g) + O2 (g) 2 SO3 (g) When 0.28 moles of SO3 (g) are removed from the equilibrium system at constant temperature: The value ofK. The value of Qc The reaction must O run in the forward direction to restablish equilibrium. O run in the reverse direction to restablish equilibrium. O remain the same. It is already at equilibrium. The concentration of O, willarrow_forwardConsider the reaction CaCO3(s)→CaO(s)+CO2(g).Estimate ΔG∘ and if they will be spontaneous for this reaction at 1475K, 1100K, & 295K. (Assume that ΔH∘ and ΔS∘ do not change too much within the given temperature range.)arrow_forwardFor the following reaction the Kp is 0.254 qt 1000 degree celcius. At equilibrium, the pressure of B is 1.55atm. What is the parial pressure of C? A(s)+2B(g)---->C(g)arrow_forward
- For each of the rate laws below, what is the order of the reaction with respect to the hypothetical substances X, Y, and Z? What is the overall order? (For each answer, enter an exact number as an integer or decimal.) (a) ✗ Y rate = k [X][Y]² [Z] 2 Ꮓ Overall (b) -1 rate = k [X]0.5 [Y]1.5 [Z]¯1 X X 2 Y Z Overall [Z]³ (c) rate = k (X) 2 ☑> Y 2 Ꮓ Overallarrow_forwardCalculate the standard free energy for the reaction at 312.0°C. (you may use any valid technique - there may be more than one way to do a question like this): Al2O3(s)) + 3 H2(g) → 2 Al(s) + 3 H2O(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The