
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:try 2 (Worksheet) 4 0
Practice Problems
Open with
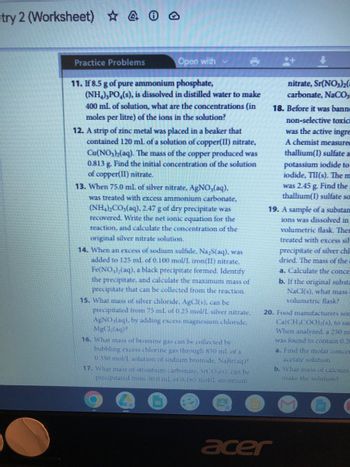
11. If 8.5 g of pure ammonium phosphate,
(NH₂),PO (s), is dissolved in distilled water to make
400 mL of solution, what are the concentrations (in
moles per litre) of the ions in the solution?
12. A strip of zinc metal was placed in a beaker that
contained 120 mL of a solution of copper(II) nitrate,
Cu(NO3)2(aq). The mass of the copper produced was
0.813 g. Find the initial concentration of the solution
of copper(II) nitrate.
13. When 75.0 mL of silver nitrate, AgNO₂(aq),
was treated with excess ammonium carbonate,
(NH4)2CO3(aq), 2.47 g of dry precipitate was
recovered. Write the net ionic equation for the
reaction, and calculate the concentration of the
original silver nitrate solution.
14. When an excess of sodium sulfide, Na,S(aq), was
added to 125 mL of 0.100 mol/L iron(II) nitrate,
Fe(NO3)₂(aq), a black precipitate formed. Identify
the precipitate, and calculate the maximum mass of
precipitate that can be collected from the reaction.
15. What mass of silver chloride. AgCl(s), can be
precipitated from 75 ml of 0.25 mol/L silver nitrate,
AgNO3(aq), by adding excess magnesium chloride,
MgCl₂(aq)?
16. What mass of bromine gas can be collected by
bubbling excess chlorine gas through 850 mL of a
0.350 mol/L solution of sodium bromide. NaBr(aq)?
17. What mass of strontium carbonate, SrCO,(s). can be
precipitated trom 50.0 mL of 0.165 mol/L strontium
EU
nitrate, Sr(NO3)2(
carbonate, NaCO3
18. Before it was banne
non-selective toxic
was the active ingre
A chemist measured
thallium(I) sulfate a
potassium iodide to
iodide, TII(s). The m
was 2.45 g. Find the
thallium(I) sulfate so
19. A sample of a substan
ions was dissolved in
volumetric flask. Ther
treated with excess sil-
precipitate of silver chl
dried. The mass of the
a. Calculate the conce
b. If the original substa
NaCl(s), what mass
volumetric flask?
20. Food manufacturers som
Ca(CH₂COO) (s), to sat
When analyzed. a 250 m
was found to contain 0.28
a. Find the molar concer
acetate solution.
acer
b. What mass of calcium
make the solution?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Suppose 0.274 g of lead(II) acetate is dissolved in 100. mL of a 23.0 mM aqueous solution of ammonium sulfate. Calculate the final molarity of acetate anion in the solution. You can assume the volume of the solution doesn't change when the lead(II) acetate is dissolved in it.arrow_forwardPotassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHC8H4O4. This is often written in shorthand notation as KHP.How many grams of KHP are needed to exactly neutralize 39.7 mL of a 0.105 M potassium hydroxide solution ?arrow_forwardPotassium hydrogen phthalate is a solid, monoprotic acid frequently used in the laboratory to standardize strong base solutions. It has the unwieldy formula of KHC3H,04. This is often written in shorthand notation as KHP. What volume of a 0.438 M sodium hydroxide solution is needed to exactly neutralize 3.48 grams of KHP ?arrow_forward
- If 23.1 g of NaOH (MM = 40.00 g/mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of NaOH in the resulting solution? What quantity in moles of NaI are in 55.8 grams of NaI ?arrow_forwardA saline solution contains 0.770 g of NaCl (molar mass = 58.55 g/mol) in 118 mL of solution. The concentration is 0.112 M. What is the concentration of Na and Cl ions in this solution given in molarity and rounded to 3 significant figures?arrow_forwardA sodium sulfate solution is added to a 25.0 mL sample of a solution containing Ba2+ ions until no more precipitate formed. The precipitate (BaSO4) is collected, dried, and weighed. If 1.59 g of the precipitate are obtained, what is the concentration (in M) of Ba2+ ions in the 25.0 mL sample? Your answer should have 3 sig. figs. Molar Masses: (Na2SO4: 142.05 g/mol) (Ba2+: 137.3 g/mol) (BaSO4: 233.37 g/mol)arrow_forward
- 4. In the reaction of Zinc(II) carbonate and HCl forming Zinc(II) chloride, water and CO2 there was 45g Zinc(II) carbonate and it took 181mL of HCl solution to completely react. Find the molarity of the HCl solution used.arrow_forwardA student prepares a solution by dissolving 9.23 g of solid KOH in enough water to make 300.0 mL of solution. Calculate the molarity of K+ ions in this solution.arrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO3)2(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 17.07 g PbCl2(s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO3)2(aq) solution.arrow_forward
- 8. A chemist wants to make 100 of a 0.15 M AlCl 3 (aq) solution. How many grams of aluminum chloride should the chemist use?arrow_forwardOne 240. mL serving of ChemColaTM contains 54.0 mg phosphorus (which is found in the phosphoric acid, H₂PO, in ChemCola). What volume (in 3 mL) of 0.0850 M NaOH is needed to neutralize the H₂PO in one serving of ChemCola™? mL NaOHarrow_forwardA chemist prepares a solution of iron(II) bromide (FeBr,) by measuring out 97.1 mg of FeBr, into a 100. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Br anions in the chemist's solution. Be sure your answer is rounded to 3 significant digits. olo mol x10 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY