
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
find major product
Please provide only typed answer solution no handwritten solution needed allowed...
Thanks
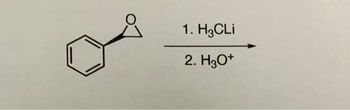
Transcribed Image Text:1. H3CLi
2. H3O+
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work..don't give Handwritten answer..don't use Ai for answering this..give correct solutionarrow_forwardhiine teaching and I... C In A Series Of Experiment= Paraphrasing .ewriting Tool Car note CAU EMAIL CANVAS Pirate Ship MYJSCC BLACKBOARD Vacancies [References] Use the References to access important values if needed for A student measures the molar solubility of nickel(II) hydroxide in a water solution to be 4.22x10-6 M. Based on her data, the solubility product constant for this compound is Submit Answer Try Another Version 3 item attempts remaining DEC 10 80 000 000 F2arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- can someone please help me with my chemistry lab. chemistry is so extensive and i am overwhelmed. i have no idea at this point i am so confused. i will be glad to rate you if you can please help me. i have attached the lab and the sheet i need help with. please show how you solved. PLEASE HELP ME!arrow_forwardThe sketch that indicates the change that occurs when 1.0 mol/ LH,PO,tan is added to 20 mL of 1.0 moVL NH, (ae) is 14 12 10 6. 2- 20 Volume of acld added (mL) 10 30 14 12 10 10 20 Volume of acld added (mL) 30 14 12 10 10 20 30 Volume of acid added (ml) 14 12 10 20 Volume ol acid added mt.) 10arrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward
- in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forward20 E The volume of water needed to dissolve 0.0620 grams of copper(II) carbonate is Assume no volume change upon addition of the solid. Submit Answer $ 4 R % 5 [Review Topics) References) Use the References to access important values if needed for this question. Retry Entire Group 8 more group attempts remaining Cengage Learning Cengage Technical Support T A 6 MacBook Pro Y & U ▶II * 00 8 1 ( 9 ) 0 P IL. Previous Email Instructorarrow_forwardOpenCCC: Interna... SForm N-648 New Tab + Downloads P lapd G My Account Apply Now Join... Los Angeles Depa.. Initial Knowledge Check Question 16 Magd Sulfuric acid (H,SO,) is a polyprotic acid. Write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water. FL O I Don't Know Submit 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibility D? 國 TOOO FES 23 14 tv Aa MacBook Air DII DD F9 F10 F11 1IIarrow_forward
- Pls help ASAP. Pls show all work and calculations.arrow_forwardin text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all working!!!!!!!arrow_forwardHelpppsppspspppspspspslslsls A. Are the coefficients from balanced equations B. Do not appear in a mass action expression C. Can be expressed in terms of concentrations or partial pressures D. Is signified by brackets E. Must be expressed in terms of concentrationsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY