
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
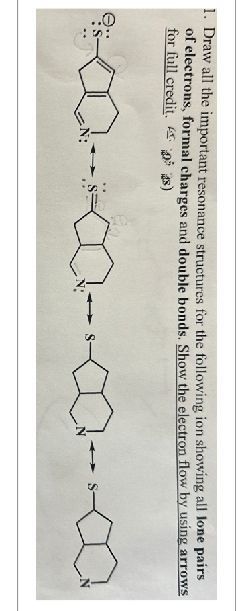
Transcribed Image Text:1. Draw all the important resonance structures for the following ion showing all lone pairs
of electrons, formal charges and double bonds. Show the electron flow by using arrows
for full credit.)
མ་
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Formal charge is (select all that are true) equal to the number of valence electrons minus half the number of bonding electrons minus the number of nonbonding electrons. equal to number of valence electrons minus number of bonds minus number of nonbonding electrons. the difference between the number of valence electrons of each atom and the number of electrons the atom is associated with. the charge assigned to an atom in a molecule.arrow_forwardCalculate the formal charge on each of the atoms in the Lewis structure given. Be sure to answer all parts. Oxygen: Sulfur: Left chlorine: Right chlorine: :CI-S-CI: Thionyl chloridearrow_forwardDraw a resonance structure of the compound shown below, called 2-heptanone, which is found in some kinds of cheese. en Draw curved arrow(s) on the initial structure to show the expected resonance. Modify the second structure given to draw a new resonance structure, including any relevant formal charges. Use the + and - tools to add/remove charges to an atom, and use the single bond tool to add/remove double bonds. H3C CH3 Edit Drawing H3C CH3arrow_forward
- Draw Lewis structures and resonance structures (if any) that satisfy the octet rule for each of the following ions with all valence electrons and formal charges clearly noted.a) NH2- b) NO2- c) ClO- d) HCOO- (formate) e) BH4- f) CH3CH2CO2H g) O3 h) CH2N2arrow_forwardPls help ASAP.arrow_forwardPls help ASAP.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning