
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
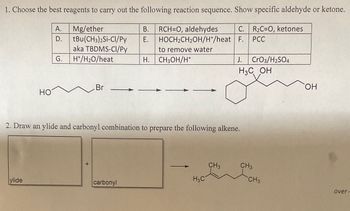
Transcribed Image Text:1. Choose the best reagents to carry out the following reaction sequence. Show specific aldehyde or ketone.
RCH=O, aldehydes C. R₂C=O, ketones
HOCH₂CH₂OH/H+/heat F. PCC
HO
ylide
A.
Mg/ether
D. tBu(CH3)2Si-Cl/Py
aka TBDMS-CI/Py
H+/H₂O/heat
G.
Br
B.
carbonyl
E.
to remove water
H. CH3OH/H+
2. Draw an ylide and carbonyl combination to prepare the following alkene.
H3C
J. CrO3/H2SO4
H3C OH
CH 3
CH3
CH3
OH
over-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- TReferences] Specify the reagent you would use in each step of the following synthesis: Br step 1. step 2 Reagents Available a. LIAIH4 f. PBr3 k. CH;CH,MgBr b. H2SO4 g. Dess-Martin periodinane (DMP) I. CeH;MgBr (phenylmagnesium bromide) c. HCI h. NaH d. HBr i. NAOH m. (CH3)2CHMgBr n. Cro3 e. SOCI, j. CH3MGBR Write the letters of the reagents in the boxes below. Reagent for step 1 Reagent for step 2arrow_forward26. Give the major organic product of each reaction. Indicate if a racemic mixture is expected. Br OH Br₂ Br₂ a. b. H₂O raumic racenie CH30 OH Br2 ICHS a. Br2, H₂O C. d. CH3OH b. NaH Br racemic racer Cl e. Cl₂ JOH OH f. a. Cl₂, H₂O b. NaH racearrow_forwardWhat are the reagents needed to complete this reaction? a. Hz/ Lindlar's cataly st b. Li, E+NH2,-78° follo wed by NHy CI wash l1owe C. Hz/ P+ d. Na BHy , Me OHarrow_forward
- 7. Predict the major product or provide the reagents in the following reactions. Be sure to show stereochemistry if necessary. ? CrO3, H2SO4, H20 a. Br HO. acetone b. 1. MgBr 2. H3O* workup с. NABH4 H. MeOH d. Br Mg, Et,0 MeOHarrow_forwardX app.101edu.co I UL Week 7: Panonto CH3Br ||| DII Br || 4x X UL LTU 7-1A F4 Rank these alkyl halides in order of decreasing reactivity in an SN2 reaction. 4 Br F5 40 X Question 1 of 24 F6 O C|Chegg.com S F7 A) III < | < || B) || < | < ||| C) ||| < || < | D) | < || < ||| E) | < ||| < || PrtScn X + FB Home F9 A End F10 Tp S # ENG @ 10 PgUp 611 0 Show 10/arrow_forwardChoose the best reagents to complete the reaction shown below. HBr А 1. LIAIHA 2. H30* 1. NaBH4 2. H30* 1. H30* D 2. PhMgBr 1. PhMgBr E 2. H30* Version: 1.0.94 + production B.arrow_forward
- Specify the reagent you would use in each step of the following synthesis: OH step 1 step 2 Reagents Available a. LIAIH4 f. PBr3 k. CH;CH,MgBr b. H2SO4 g. pyridinium chlorochromate (PCC) I. CęH5MgBr (phenylmagnesium bromide) c. HCI h. NaH m. (CH3)2CHMgBr d. HBr i. NaOH n. Cro3 e. SOCI2 j. CH3M9B Write the letters of the reagents in the boxes below. Reagent for step 1 Reagent for step 2arrow_forwardPlease helparrow_forwardDraw the product of each reagnarrow_forward
- Starting materials Br Reagents OH 1 H3C OH 2 Br 6 Br 7 OH OH TOH OH 8 5 H a Mg / dry ether e Aqueous H2SO4 at reflux i KMnO4 / H3O+ b 1. CO2 2. acidic workup of Conc. HCI or HCI (gas) j Na2CrO4 / aqueous H2SO4 C NaCN/THF or DMF g PBr3 K 1. BH3/THF 2. H₂O2/aq. NaOH d NaCN/dil. aqueous H2SO4 h KOH alcohol |arrow_forward12. Which reagents/conditions would lead to the transformation depicted below in high yield? a. NaOMe, MeOH b. MeOH, H₂O* c. NaOEt, EtOH 13. In the structure of 2-fluoropropanoic acid shown, the oxidation number on C1 is the oxidation number on C2 is a. +1, +2, -3 b. -3, +1, +3 d. NaOH, H₂O e. two of these , and the oxidation number on C3 is, c. 0, 0, 0 d. +3,0,-3 e. +2,0, -2 ??? OH 14. What will be the outcome of the following reaction sequence? B. 1. NaOEt, EtOH 2. PCC 3. isopropylMgBr 4. H₂0¹ ??? a. 2-ethyl-3-isopropyl-2,3-dimethyloxirane b. 2,3,4-trimethyl-3,4-epoxyhexane c. 1,2,3,4-tetramethyl-2,3-epoxypentane d. Both a and b are suitable. e. All of the above are correct. OH 15. What is a suitable IUPAC name for the structure shown at the right? OH D. F H OH LOH CH3 3 OHarrow_forwardSpecify the reagent you would use in each step of the following synthesis: CI step 1 step 2 H. Reagents Available a. LIAIH4 f. PBr3 k. CH;CH,MgBr b. H2SO4 g. Dess-Martin periodinane (DMP) 1. CgHgMgBr (phenylmagnesium bromide) с. НС! h. NaH m. (CH3)2CHMgBr d. HBr i. NaOH n. Cro3 e. SOCI, j. CH3MGB Write the letters of the reagents in the boxes below. Reagent for step 1 Reagent for step 2| An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY