
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
#10).
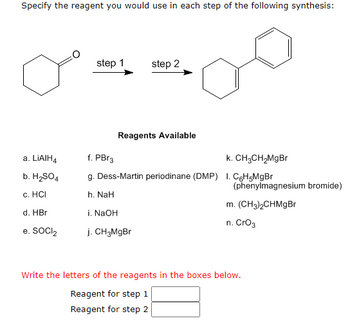
Transcribed Image Text:Specify the reagent you would use in each step of the following synthesis:
a. LIAIH4
b. H₂SO4
c. HCI
d. HBr
e. SOCI₂
step 1
step 2
Reagents Available
f. PBr3
k. CH3CH₂MgBr
g. Dess-Martin periodinane (DMP) 1. C6H5MgBr
h. NaH
i. NaOH
j. CH3MgBr
(phenylmagnesium bromide)
m. (CH3)2CHMgBr
n. CrO3
Write the letters of the reagents in the boxes below.
Reagent for step 1
Reagent for step 2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- bard 1 ! New folder M Reading Mode: 1.5... M Gmail ض A- ش X Course Home Z~ is 2 https://openvellum.ecollege.com/course.html?courseld=17485264&OpenVellumHMAC-453c4e9377366ab5782f501c5246fbe0#10001 ▾ 2 Part A alt W= VA 3 Part B Complete previous part(s) S- = Part C Complete previous part(s) Part D Complete previous part(s) X a 2,4-floro, 5-methyl he XI Determine if each of the following cycloalkanes and alkenes can exist as cis-trans stereoisomers. Drag the appropriate items to their respective bins. Cis-trans isomers are possible. Submit Request Answer XO, F3 # 3 E- [» "! D[ LS ra. YouTube 4 C { $ R 24 F1 H₂C. Maps FS ▬▬▬ ▬▬ % 2,4-fluoro,5-methyl h X CH₂ 5 V} TY CH₂ ف GY Br. CH₂ 6 Ą Cis-frans isomers are not possible. XXX YI Copyright © 2022 Pearson Education Inc. All rights reserved. Terms of Use | Privacy Policy Permissions | Contact Us | BY Y 5-Methyl-2,4-hexane X m.__ CH₂CH₂CH-CHF & 7 Hi FB U E W J NI Pearson ی 8 F9 DELL Reset Help Q trimethyl - prt sc 1 ÷ D MA ( 9 к. 30 F10 ن O…arrow_forward5. You have seen Lineweaver Burke and MM Plots. Another way to express kinetic data is to make an Eadie Hofstee Plot (Vo on the Y-axis vs. Vo/[S]) on the X-axis a) Rearrange MM equation to give Vo as a function of Vo/[S] b) c) What do the slope, y-intercept, and x-intercept represent on this plot? Sketch a single Eadie Hofstee plot with three lines that show: no inhibition, competitive inhibition, and uncompetitive inhibitionarrow_forwardMuch of Earth's history can be unraveled studying sulfur. Sulfur can be used as "geothermometer". The ratios of stable sulfur isotopes change with the temperature of Earth's processes. For example, igneous systems deep inside the Earth occur at very high temperatures, hydrothermal systems occur at intermediate conditions, and sedimentary rock weathering occurs at low temperatures. The composition of stable sulfur isotopes varies across the 1000 degree temperature range. 10. How many peaks would be seen in the mass spectra for such a study? Make a basic sketch (bar chart) a.m.u. vs % natural abundance and label each peak's a.m.u. and % natural abundance.arrow_forward
- O (81) Drake - In The Bible (Official x Answered: Drag the text element X Chapter 3 Competency 5 G molar mass of fe - Google Search x + -> A cloud.scorm.com/ScormEnginelnterface/defaultui/player/legacy.html?configuration=CbMtix7R173giv8Jlq2qNte5-N4X7iO1ARnDq98KvBHVj4XKlo1rLn9XYyotbblQjPsc2h... * I Apps © Google MyMasteryPath co Welcome to LSU M.. P MasteringBiology E FRENCH ALEKS – Adaptive L. Pt Periodic Table - Pta. Other bookmarks E Reading list Chapter 3 Competency 5 POWERED BY THE RUSTICI EN PREVIOUS NEXT CLOSE ITEM RETURN TO LMS Page 8 of 35 Your Learning Points 4 Osiagwu, Chiemeka K Iron metal reacts with elemental sulfur to produce iron (III) sulfide. What mass of iron is required to react with 13. 61 g of sulfur? (A) 79. 99 g Check your balanced reaction 2 Fe(s) + 3 S(s) -> Fe,S, (s) (В) 53. 33 g (C) 61. 33 g (D) 15. 8 g (E) 35. 55 g 9:27 PM O Type here to search A 4) 9/25/2021 1Oarrow_forwardHow do I draw the arrows for each step?arrow_forwardMicrosoft C A student proposes the following Lewis structure for the nitrite W W Microsoft [³0-№=0] esc Microsoft 6.52.210... Assign a formal charge to each atom in the student's Lewis structure. atom left O ! 1 N Explanation right O BO Q formal charge NE 2 0 U Check 8,998 X > W 280 Q # 3 E X 17 C DEC 8 $ 4 S BO R ол оро % (NO₂) io 5 tv ♫ T ion. < 6 ∞ g Y 7 2022 McGra 1 Uarrow_forward
- 12:49 1 Send a chat Paragraph Styles Edit I 1 2 . 3 4 L..5 . 6.. I 7.. Problems from Periods 18-19: 1. Gasoline combustion engines run on the following balanced chemical reaction between octane (CH) and oxygen (0.): 20,H1 (g) + 250, (g) 16CO, (g) 18H,0 (g) A small car's gas tank holds 34292 grams (75.6 |bs) of gasoline. a. How many grams of the greenhouse gas CO, are produced by one tank of gasoline? Convert that number to pounds (Ibs). b. How many grams of O, are necessary to completely react with one tank of gasoline? CPacusarrow_forward12. A buret is filled with deionized (DI) water and the stopcock is used to adjust the meniscus of the water in the buret to an initial value of 1.06 mL. The stopcock is opened and the DI water is dispensed in a flask. The stopcock is closed and the final buret reading is 15.72 mL. The volume of DI water (mL) in the flask is 14.66 35.34 16.8 14.7arrow_forwardΣ Mail - Shakyra Adams-Outlook X Question 4-Questions: Ch 5 Gas x t Watch A Doll's House (1973)- Fr > heducation.com/ext/map/index.html?_con%3Dcon&external.browser=0&launchUrl=https%253A%252F%252Felearning.uaptc.e March 15-... a Study: Rap Music Li. O Positive Impacts-i.. Hb Week 14 (April 19-2.. Hb Blackboard Learn Imagery essay Ch 5 Gases i 1. 3 attempts left Check my work A sample of helium gas has a volume of 3.24 L at 25°C. What volume will it oCcupy at 286 K if the pressure and number of mol are constant? Enter your answer in the provided box. L. Pping < Prev 4 of 15 acerarrow_forward
- 1.19 The lattice constant of a single crystal is 4.73 Å. Calculate the surface density (#/cm²) of atoms on the (i) (100), (ii) (110), and (iii) (111) plane for a (a) simple cubic, (b) body-centered cubic, and (c) face-centered cubic lattice.arrow_forward12:50 ← d2fcc1b0-17ba-42ff-a... 15/24 SCIENCE (086) 9 જે SCIENCE 224 Science Project Create a comprehensive and informative project on ' Natural resources' in the form of an artistically crafted, decorated project file Include pictures, illustrations,examples, surveys, advertisements, newspaper cuttings and headings etc in your project to make your project appealing, relevant, easy to understand and memorable Keypoints . Introduction to Natural Resources • Air pollution and its causes Water pollution and its causes Soil pollution and its causes . Biogeochemical Cycles 1. Oxygen Cycle 2. Carbon Cycle 3. Nitrogen Cycle 4. Water Cycle Rain and effect of acid rain . Green-house Effect Ozone Layer and reason for ozone depletion Assignment Sheet 1.Look at Fig. 1.1 and suggest in which of the vessels A,B, C or D the rate of evaporation will be the highest? Explain. PDF to Long Images PDF to Images =arrow_forwardA Aa A Y F 3 States) Text Predictions: On 13 C 14 101 !!! M $ 4 ET 垣~ Paragraph What is the difference between a cylinder and a test tube? B 1.The cylinder is made of glass and the test tube is made of glass. f5 Accessibility: Good to go % 5 3.The cylinder is the same as the test tube, it's just another name for it. V 2.The cylinder contains dimensions to measure volume and the test tube is to observe reactions. f6 4.The test tube comes in a single measure (25 ml) and the test tube can come in different volumes (25- 200 ml for example). 5 A 6 P 17 Normal & 7 18 7 hp No Spacing O 19 Styles 8 Heading 1 f10 f17 .. V F12 5 فکر Ed 82°F Mosarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY