
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
can you help to confirm the major organic prodcut of rx
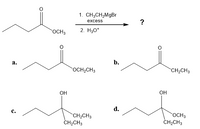
Transcribed Image Text:1. CH3CH2MgBr
excess
OCH3
2. H30*
а.
b.
OCH2CH3
CH2CH3
OH
OH
d.
C.
CH2CH3
CH2CH3
OCH3
ČH;CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi! I am trying to find the synthetic route for this question. Would really appreciate some help. Thank you so much! If you could include an explanation that would also be great! Thanks again!!arrow_forwardMENH2 SO3ME MеHN. NEC- CI NEC a. Draw a reasonable arrow-pushing mechanism for this transformation. CIarrow_forwardCould you write out the mechanism for this sequence?arrow_forward
- 1. propose a synthetic route to this molecule that includes at least one C-C bond formation step. 2. draw a arrow pushing mechanism for this moleculearrow_forward1. LIAIHY 2.H3O+ work up Hi Would it be...? Primary Ale o Secondary Ako Ketone t Tertiary Alte Aldehyde Carboxylic Acid. Choose as many opply ? thatarrow_forwardLab J Exp 17: Transfer Hydrogenation Data Analysis Packet 1. Predict the product of the reaction you are doing in this lab and come up with a logical mechanistic sequence (i.e., sequential dehydrogenations and hydrogenations). Remember, aromaticity is the driving force for this transfer hydrogenation. You do not need to add curved arrows, but you should have reasonable intermediates. carvone Pd/C tautomerization dehydrogenation final product hydrogenation + H₂arrow_forward
- Attached 1st Reaction was performed in the Synthesis of Dihydrosafrole via Friedel-Crafts Acylation & Reduction Experiment resulting in following IR spectra. Can you analyze it and fill the following table? Are their any impurities found? Peak Position cm-1 Bond Type Functional Grouparrow_forwardPlease answer fast i give you upvote.arrow_forwardDraw the correct product of the given reactions. Then identify the synthetic trap and explain why the proposed rxn with the desired product would not work.arrow_forward
- Draw the x anomer product of this reaction. Ignore inorganic byproducts. HO HOH OH HO H H H3O+ Drawing く H -OCH3 Q H Atoms, Bonds and Rings Charges Tap a node to see suggestions. H H H H H H H H Undo Reset Remove Done Drag To Panarrow_forwardConsider the synthetic sequence shown. Identify the reagents for all three steps. Draw the structures of organic compounds A and B. Omit byproducts.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY