
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Draw the correct product of the given reactions. Then identify the synthetic trap and explain why the proposed rxn with the desired product would not work.
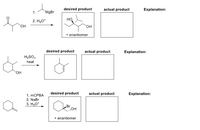
Transcribed Image Text:desired product
actual product
Explanation:
1.
MgBr
2. H3O*
HO
он
он
+ enantiomer
desired product
actual product
Explanation:
H2SO,
heat
HO,
desired product
actual product
Explanation:
1. MCPBA
2. NaBr
3. H3O*
Br
OH
+ enantiomer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the aldol or enone below. You do not have to consider stereochemistry. Draw the enolate ion in its carbanion form. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple reactants using the + sign from the drop-down menu.arrow_forward$ 12.70a1 1) Excess MeMgBr 2) H₂O* HO Mg T Br X₂ Add curved arrow(s) to complete step 1 of the mechanism. Modify the given drawing intermediate that is formed in this step. Use the single bond tool to interconvert single Edit Drawing OHarrow_forwardDraw the major organic product or products for this reaction. Be sure to include stereochemistry where appropriate. Assume it takes place in the most appropriate solvent. Br: Please answer fast I give you upvote.arrow_forward
- Styrene can be converted to the aldehyde show below in a number of ways. Draw one of these. Be explicit with each synthetic step you propose. You do not need to draw mechanisms. CHOarrow_forwardDraw the major product of this SN1 reaction. Ignore any inorganic byproducts.arrow_forward10) Which compound undergoes SN1 reaction faster? Br Br Br Br 6666 A Barrow_forward
- A 150.0-g sample of a metal at 88.5°C is added to 150.0 g of H₂0 at 17.9°C. The temperature of the water rises to 21.9°C. Calculate the specific heat capacity of the metal, assuming that all the heat lost by the metal is gained by the water. J°C-1g-1 Need Help? Read It Supporting Materials Periodic Table Watch It Constants & Factors Supplemental Dataarrow_forwardDraw the structure of each of the organic precursors in the following two-step synthesis.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY