
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Calculate the molecular weight (g/mol) of each pictured compound
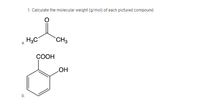
Transcribed Image Text:1. Calculate the molecular weight (g/mol) of each pictured compound
H3C
CH3
a.
СООН
HO
b.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- 24) Calculate the molar mass of the following compound: ammonia, NH3 A) 15.02 g/mol B) 17.03 g/mol C) 14.01 g/mol 25) Calculate the molar mass of the following compound: boric acid, B(OH)3 A) 16.00 g/mol B) 10.81 g/mol C) 61.83 g/mol 26) Determine the number of moles of compound within 25.0 g of propylene, C3H§ A) 1.68 mol of C3H6 B) 42.08 mol of C3H6 C) 0.594 mol of C3H6arrow_forwardComplete the table below for calculating the molar mass of the compound phosphorus triiodide. Molar mass of element Mass in one mole of phosphorus triiodide Moles g/mol mol %3D g g/mol x mol %3D Molar mass phosphorus triiodide = g/molarrow_forwardWhat are the molecular weights (molar mass) of the following compoundsarrow_forward
- Find the moles of NH4NO3 in this sample molecular weight- 80.05 g/mol mass- 2.0012 garrow_forwardA sucrose sample, with a mass of 4.601 g, contains 42.12 % carbon, 6.48 % hydrogen, the remainder being oxygen. What is the molecular formula of glucose, assuming the molecular weight is 342.2 g/mol?arrow_forwardWhat is the Molar Mass or Molecular Weight for H2O? 20.0 g/mol O 18.02 g/mol O 2.02 g/mol O 19.0 g/molarrow_forward
- What factors during the reducing bezil experiment caused the percent yield to be different than 100%? What impurities may be present in the final product and how does that effect the percent yield determination?arrow_forwardWhat is the molar mass of Ca(ClO4)2? 171.6 g/mol 203.6 g/mol 239.0 g/mol 279.1 g/molarrow_forwardIf the empirical formula molar mass of an unknown chemical is 58.08 g/mol, which of the following could NOT be the molar mass of the compound? 58.08 g/mol 87.12 g/mol 116.16 g/mol 174.24 g/mol 290.4 g/molarrow_forward
- 1. Determine the empirical formula of a compound that is 15% C, 5.0% H, and 80% S in mass. molar mass: C: 12.01g/mol; H:1.008g/mol, S:32.07g/mol 2. Complete the combustion of 4.20g of hydrocarbon( contains only C and H) produced 12.9g of CO2 and 5.96g of H2O. What's the empirical formula for hydrocarbon? What's the molecular formula of hydrocarbon? The molar mass of the compound is 57g/mol.arrow_forwardIf the empirical formula of a compound is P2O5, what could be a possible molar mass of the molecular compound? 165 g/mol 55 g/mol more than one of these could be a valid molar mass of the compound none of these could be a valid molar mass of the compound 275 g/molarrow_forwardCyanide chloride(C15H11O6Cl) contains the cyanidin ion. Calculate the number of moles of cyanidin chloride equivalent to 7.2 mgarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY