
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
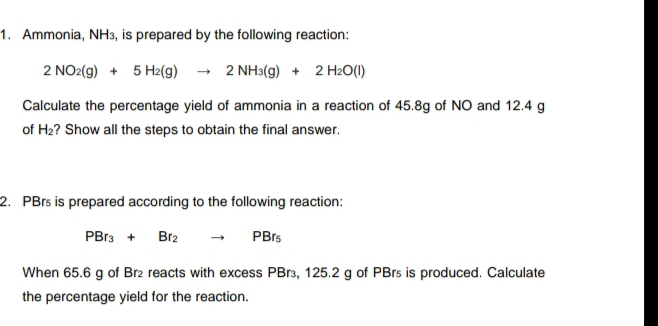
Transcribed Image Text:1. Ammonia, NH3, is prepared by the following reaction:
2 NO2(g) + 5 H2(g) - 2 NH3(g) + 2 H2O(I)
Calculate the percentage yield of ammonia in a reaction of 45.8g of NO and 12.4 g
of H2? Show all the steps to obtain the final answer.
2. PBrs is prepared according to the following reaction:
PBr3 +
Br2
PBrs
When 65.6 g of Br2 reacts with excess PB13, 125.2 g of PBrs is produced. Calculate
the percentage yield for the reaction.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- 4. Chemistry is the science dealing with: A) The structure and composition of substances B) Changes in composition C) Mechanisms by which these changes occur D) All of the abovearrow_forwardPlease answer in tipping formatarrow_forward7.How much Insecto 4 E would be needed to make up 150 gallons of a 2% spray solution?arrow_forward
- O Assignment Score: one Up 70.6% O Resources O Hint Check Am Draw 3-ethylhexanoic acid. Draw the structure in line-bond form. Select Draw Rings More Erase Harrow_forwardLuLu mixes a solution of vinegar for a homemade salad dressing she is making. She makes 300 mL of vinegar by mixing 135 mL acetic vinegar with water. What is the percent by volume of the solute? Use details to support your answer.arrow_forwardLabel the parts of the equation correctly. 2 H2 + 0,– 2 H,0 :: Products :: Reactants 6 7 8 9.arrow_forward
- Predict the products of the following reaction. If no reaction will occur, use the NO REACTION button. Be sure your chemical equation is balanced! →+AlsHNO3aqarrow_forwardMethane, CH4, is the simplest hydrocarbon molecule and in its natural state is a gaseous substance. If you had 64 grams of methane, how many moles of methane would that be? Group of answer choices A. 1 B. 2 C. 4 D. 8arrow_forwardhow many mL of pure alcohol are in a 250 mL glass of wine that 14 percent alcohol by volumearrow_forward
- 5. A catalyst is a substance that: (a) Increases the rate of a reaction (b) Becomes part of the products (c) Decreases the activation energy (d) All of the abovearrow_forwardQuestion 30 How many grams are in 5.60 x 1024 molecules of NaCI? Previousarrow_forwardAll of the following are bases except A. NaOHB. Al(OH)3C. HBrD. Ca(OH)2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON