
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Solve the following problem. Show your solution.
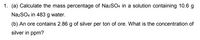
Transcribed Image Text:1. (a) Calculate the mass percentage of NazSO4 in a solution containing 10.6 g
NazSO4 in 483 g water.
(b) An ore contains 2.86 g of silver per ton of ore. What is the concentration of
silver in ppm?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Silver ions can be found in some of the city water piped into homes. The average concentration of silver ions in city water is 0.028 ppm. (a) How many milligrams of silver ions would you ingest daily if you drank eight glasses (eight oz/glass) of city water daily? (b) How many liters of city water are required to recover 1.00 g of silver chemically?arrow_forwardFluoridation of city water supplies has been practiced in the United States for several decades. It is done by continuously adding sodium fluoride to water as it comes from a reservoir. Assume you live in a medium-sized city of 150,000 people and that 660 L (170 gal) of water is used per person per day. What mass of sodium fluoride (in kilograms) must be added to the water supply each year (365 days) to have the required fluoride concentration of 1 ppm (part per million)that is, 1 kilogram of fluoride per 1 million kilograms of water? (Sodium fluoride is 45.0% fluoride, and water has a density of 1.00 g/cm3.)arrow_forwardWhat is the mole fraction of H 2 S O 4 in a solution containingthe percentage of sulfuric acid and water shownin Figure 14.25?arrow_forward
- Calculate the molarity of AgNO3 in a solution prepared by dissolving 1.44 g AgNO3 in enough water to form 1.00 L solution.arrow_forwardPredict the results of passing a direct electrical current through (a) molten NaBr, (b) aqueous NaBr. and (c) aqueous SnCl2.arrow_forwardWhen 85.0 mL of 0.250 M Ba(OH)2 solution is added to 85.00 mL of 0.250 M Al (NO3)3 solution, a white gelatinous precipitate of Al(OH)3; is formed. Assuming 100% yield, (a) what mass (in grams) of Al(OH)3 is formed? (b) what is the molarity of each of the ions Ba2+, OH-, Al3+, NO3- in the resulting solution?arrow_forward
- A certain grade of steel is made by dissolving 5.0 g of carbon and 1.5 g of nickel per 100. g of molten iron. What is the mass percent of each component in the finished steel?arrow_forwardThe concentration of sodium chloride in seawater is approximately 0.481 M NaCl. How many grams of NaCl would you need to make 1.0 L of water with the same concentration of NaCl as seawater?arrow_forwardWhat is the total molar concentration of ions in 34.49mL of 0.2192M Ca3(PO4)?arrow_forward
- 3 g BaI2 is dissolved in 145.0 g of H2O. Calculate the percentage concentration (by mass) of BaI2.arrow_forwardConsider a solution prepared by adding 2.70 g acetic acid, CH3CO2H, to 122.8 g of H2O. (a) what is the percent by mass concentration of acetic acid in the solution? (b) what mass of acetic acid is present in 25.0 g of this solution?arrow_forward(a) Lead azide, Pb3(N3)3 has been used as a detonator in automobile airbags. A saturated solution of lead (IV) azide contains 25 mg in 100.0 mL What is Ksp? Moles of Pb(N3)4 = folarity of Pb(N3)4 = mass molar mass 0.025 g 375.2804 g / mol = 6.66 ×10-5 mol moles volume (in L) 6.66 × 10-5 mol 1 L 1000 mL = 6.66 ×10-4 M 100 mLx The expression for the solubility product constant, Ksp iS Ksp = [Pb4+][N3-] = (s)(4s)4 = 256s5 = 256(6.66×10-4)5 = 3.3586×10-14 =3.4×10-14 How many grams of lead azide will dissolve in 500 mL of 100 M Pb(NO3)2 solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning