
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
Show work. Don't give Ai generated solution. Give detailed Solution
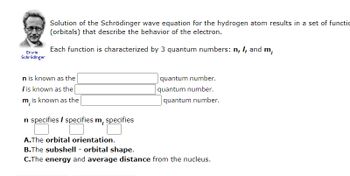
Transcribed Image Text:Erwin
Schrödinger
Solution of the Schrödinger wave equation for the hydrogen atom results in a set of functio
(orbitals) that describe the behavior of the electron.
Each function is characterized by 3 quantum numbers: n, I, and m,
n is known as the
I is known as the
m, is known as the
n specifies / specifies m, specifies
A.The orbital orientation.
quantum number.
quantum number.
quantum number.
B.The subshell - orbital shape.
C.The energy and average distance from the nucleus.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- In the Stern-Gerlach experiment, silver atoms were used. This was a good choice, as it turned out. Using the electron configuration of silver atoms, explain why silver was a good candidate for being able to observe the intrinsic angular momentum of the electron. Hint: Dont use the aufbau principle to determine the electron configuration of Ag, because its one of the exceptions. Look up the exact electron configuration in a table.arrow_forwardSome scientists study Rydberg atoms, atoms whose electron has a large value of the n quantum number. Some Rydberg hydrogen atoms may have consequences in interstellar chemistry. Predict the radius of a Rydberg hydrogen atom that has n=100.arrow_forwardHow is the Bohr theory of the hydrogen atom inconsistent with the uncertainty principle? In fact, it was this inconsistency, along with the theorys limited application to non-hydrogen-like systems, that limited Bohrs theory.arrow_forward
- In 1885, Johann Balmer, a mathematician, derived the following relation for the wavelength of lines in the visible spectrum of hydrogen =364.5 n2( n2 4) where in nanometers and n is an integer that can be 3, 4, 5, . . . Show that this relation follows from the Bohr equation and the equation using the Rydberg constant. Note that in the Balmer series, the electron is returning to the n=2 level.arrow_forwardCalculate the energies of an electron in the fourth, fifth, and sixth energy levels of the Bohr hydrogen atom.arrow_forwardConsider the orbitals shown here in outline. (a) What is the maximum number of electrons contained in an orbital of type (x)? Of type (y)? Of type (z)? (b) How many orbitals of type (x) are found in a shell with n=2? How many of type (y)? How many of type (z)? (c) Write a set of quantum numbers for an electron in an orbital of type (x) in a shell with n=4, of an orbital of type (y) in a shell with n=2. Of an orbital of type (z) in a shell with n=3. (d) What is the smallest possible n value for an orbital of type (x)? Of type (y)? Of type (z)? (e) What are the possible I and ml values for an orbital of type (x)? Of type (y)? Of type (z)?arrow_forward
- The helium ion He+ is a one-electron system whose wave functions and energy levels are obtained from those for H by changing the atomic number to Z=2 . Calculate the average distance of the electron from the nucleus in the 2s orbital and in the 2p orbital. Compare your results with those in Problem 11 and explain the difference.arrow_forwardConstruct an energy level diagram showing all orbitals for the hydrogen atom up to n=5, labeling each orbital with its appropriate quantum numbers. How many different orbitals are in each shell?arrow_forwardThe wave function of an electron in the lowest (that is, ground) state of the hydrogen atom is (r)=( 1 a 0 3 )1/2exp(r a 0 )ao=0.5291010m (a) What is the probability of finding the electron inside a sphere of volume 1.0pm2 , centered at the nucleus (1pm=1012m) ? (b) What is the probability of finding the electron in a volume of 1.0pm2 at a distance of 52.9 pm from the nucleus, in a fixed but arbitrary direction? (c) What is the probability of finding the electron in a spherical shell of 1.0 pm in thickness, at a distance of 52.9 pm from the nucleus?arrow_forward
- State how many radial, angular, and total nodes are in each of the following hydrogen-like wavefunctions. a 2s b 3s c 3p d 4f e 6g f 7sarrow_forward• use Planck’s equation to calculate the energy of a photon from its wavelength or frequency.arrow_forwardA 25-kg child is on a merry-go-round/calliope, going around and around in a large circle that has a radius of 8meters. The child has an angular momentum of 600kgm2/s. a From these facts, estimate the approximate quantum number for the angular momentum the child has. b Estimate the quantized amount of energy the child has in this situation. How does this compare to the childs classical energy? What principle does this illustrate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning