
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
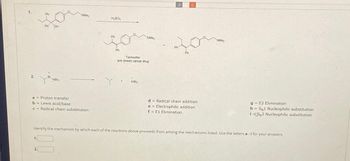
Transcribed Image Text:1.
2.
Ph
1.
Ph
2
OH
AlBrs
a = Proton transfer
b = Lewis acid/base
c = Radical chain substitution
NMe₂
H₂SO4
Ph
Ph
NMe₂
Tamoxifen
anti (brest) cancer drug
AlBr
J
Ph
Ph
C
d = Radical chain addition
e = Electrophilic addition
f = E1 Elimination
NMе₂
Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers.
g= E2 Elimination
h = SN1 Nucleophilic substitution
iSN2 Nucleophilic substitution
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 9:58 S Brown, Intro Organic Chemistry, 6e Helo I Svstem Announcements Question 4 Complete the equation by predicting the major product formed in the reaction. Note that the reaction may involve rearrangements + HCI Edit By accessing this Question Assistance, you will learn while you earn points based on the Point Potential Policy set by vour instructor Question Attempts: 0 of 2 used Eam Haimum Pints available om anwer this question co attemptarrow_forwardIdentify the mechanismarrow_forward(10) The following can undergo 1,2- or 1,4-addition reactions. Predict the major organic product from each reaction. 1. LiAlH4 2. H+ 1. CH3S- +Na 2. H+ 1. KCN 2. H+ 1. (CH3CH₂)2CuLi 2. H 1. CH3CH₂MgCl 2. H+arrow_forward
- 1. 2. 1. -CH3 H Ph HO 2. + E2 Elimination aqueous H₂SO4 a = Electrophilic addition b = C = SN1 Nucleophilic substitution f = Carbonyl nucleophilic addn Submit Answer NaOH Retry Entire Group OH CH3 d Ph = SN2 Nucleophilic substitution g e Electrophilic aromatic substitution h = Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. = 6 more group attempts remaining Nucleophilic subs at carbonyl(acyl Xfer) Conjugate (nucleophilic) addnarrow_forward10:08 AM Thu Mar 23 ← Problem 35 of 23 Draw the major product of this reaction. Ignore inorganic byproducts. + AICI 3 > @ 66% O Submitarrow_forward1. 2. Å 1. N-Br a = Proton transfer b = Lewis acid/base c = Radical chain substitution 2. H₂SO4 DMSO / H₂O OH love Br ..OH d = Radical chain addition e= Electrophilic addition f = E1 Elimination + Een N-H Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. g= E2 Elimination h = SN1 Nucleophilic substitution i = SN2 Nucleophilic substitutionarrow_forward
- 1. 2. H₂C 1. MeO 2. -CH3 H₂O a = Proton transfer b = Lewis acid/base c = Radical chain substitution H3PO4 80° MeO CH3 H₂COM₂ CH3 d = Radical chain addition e = Electrophilic addition f = E1 Elimination H₂O Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. g = E2 Elimination h = SN1 Nucleophilic substitution i = SN2 Nucleophilic substitutionarrow_forward1. 2. Br 1. + H₂O a = Proton transfer b = Lewis acid/base c = Radical chain substitution 2. NaOH Aqueous acetone OH + OH 앳 H OH + + HBr d = Radical chain addition e = Electrophilic addition f = E1 Elimination Nal Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. g= E2 Elimination h = SN1 Nucleophilic substitution i = SN2 Nucleophilic substitutionarrow_forward2. OH 1. Mg2+ Br 2. a = Proton transfer b= Lewis acid/base c = Radical chain substitution H₂SO4 140⁰ H₂SO4/ H₂O Submit Answer [Review Topics] H₂O + H₂O + Mg2+ Br d = Radical chain addition e= Electrophilic addition f E1 Elimination [References] Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. Retry Entire Group 9 more group attempts remaining HSO g=E2 Elimination h = SN1 Nucleophilic substitution i= SN2 Nucleophilic substitution Previous Next Shoarrow_forward
- 1. 2. Ph 1. Ph OH a Proton transfer b = Lewis acid/base c = Electrophilic addition 2. + Na CN NMe₂ DMF H₂SO4 Ph Ph H3C H CH3 H CN OH NM²₂ Tamoxifen anti (brest) cancer drug Ph d E1 Elimination = e = E2 Elimination Ph NMe₂ The rections above involve synthesis or reactions of alcohols and ethers. Identify the mechanism by which they proceed from among the mechanisms listed. Use the letters a- g for your answers. f = SN1 Nucleophilic substitution gSN2 Nucleophilic substitutionarrow_forward1. 2. HO a = Proton transfer b = Lewis acid/base 1. f 2. toto. H₂SO4 Submit Answer -CH3 Aqueous acetone e = Electrophilic addition f = E1 Elimination g=E2 Elimination OH H₂O Retry Entire Group 1 more group attempt remaining K ...OH = Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - j for your answers. + HO-S -CH3 h SN1 Nucleophilic substitution i SN2 Nucleophilic substitution j = Electrophilic aromatic substitutionarrow_forward1. 2. ~ 1 OH 2. Ph NaBr Submit Answer 8 a = Proton transfer b = Lewis acid/base c = Radical chain substitution d = Radical chain addition 00 100⁰ Ph H₂SO4 Retry Entire Group H₂ th polystyrene Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a answers. e = Electrophilic addition f = E1 Elimination g = E2 Elimination H₂O 8 more group attempts remaining h SN1 Nucleophilic substitution i = SN2 Nucleophilic substitution j = Electrophilic aromatic substitution Previ Email Instrarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY