
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Number 2 second part of the question asking for a. Most basic...
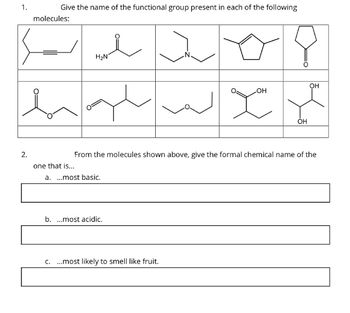
Transcribed Image Text:1.
2.
Give the name of the functional group present in each of the following
molecules:
i
H₂N
one that is...
a. ...most basic.
b. ...most acidic.
N.
From the molecules shown above, give the formal chemical name of the
C. ...most likely to smell like fruit.
OH
you
OH
OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- help with these 2 pleasearrow_forwardCan you please help me with part d?arrow_forwardA zwitterion • A. Has two or more acidic or basic functional groups. • B. Can act as both an acid and a base. • C. Bears both positive and negative charge.• D. Has two acids or two bases of different strengths.arrow_forward
- nvestigate % What term describes this particle model? A. weak base F5 C. weak acid Q Search A F6 6 V & F7 7 F8 B. strong acid D. strong base DELL 8 F9 ( 9. prtsc F10 M+ OH- A home F11 JAN end F12 xarrow_forwardI thought larger atoms are more strongly acidic? This is saying that smaller atoms are more strongly acidic.arrow_forwardPlease choose most acidic proton in photo.arrow_forward
- Tab Window Help Learning Xx AT HOMEWORK Module 9 X M hydrochloric acid Submit Answer MindTap Cengage Learning mentid=5735112480241329813180832311&elSBN=9781305862883&id=1707786068&snapshotld-33225... References Use the References to access important values if needed for this question. : Concentration Acid/Base: This is group attempt 1 of 10 tv An aqueous solution of hydrochloric acid is standardized by titration with a 0.188 M solution of barium hydroxide. X If 23.6 mL of base are required to neutralize 16.7 mL of the acid, what is the molarity of the hydrochloric acid solution? G What volume (in mL) of a 0.125 ☆ Autosaved at 10:58 PM Q A ·arrow_forwardDetermining the Acid, Base, Conjugate Acid, and Conjugate Base in a Reaction Label the acid and base, and the conjugate acid and base, in the following reaction. Use curved arrow notation to show the movement of electron pairs.arrow_forwardQuestion 1 Which of the following is/are a Bronsted base? Multiple answers are possible HBr O NH, O H2S HCO3 Question 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY