
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Q/Ture or error(false)
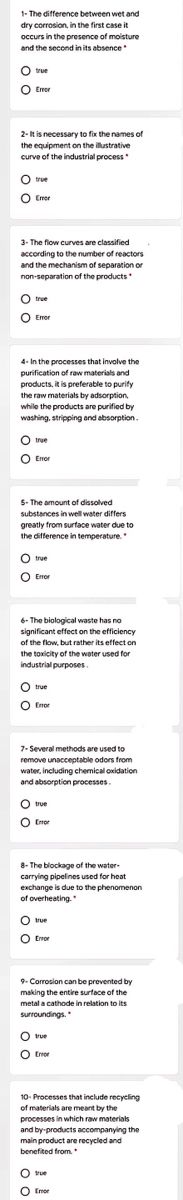
Transcribed Image Text:1- The difference between wet and
dry corrosion, in the first case it
occurs in the presence of moisture
and the second in its absence
true
O Erer
Emor
2-It is necessary to fix the names of
the equipment on the illustrative
curve of the industrial process
O true
O Emor
3- The flow curves are classified
according to the number of reactors
and the mechanism of separation or
non-separation of the products
O true
O Eror
4- In the processes that involve the
purification of raw materials and
products, it is preferable to purify
the raw materials by adsorption,
while the products are purified by
washing, stripping and absorption.
true
O Eror
5- The amount of dissolved
substances in well water differs
greatly from surface water due to
the difference in temperature.
O true
O Error
6- The biological waste has no
significant effect on the efficiency
of the flow, but rather its effect on
the toxicity of the water used for
industrial purposes.
O true
O Enor
7- Several methods are used to
remove unacceptable odors from
water, including chemical oxidation
and absorption processes.
O true
O Enor
8- The blockage of the water-
carrying pipelines used for heat
exchange is due to the phenomenon
of overheating.
O true
O Error
9- Corrosion can be prevented by
making the entire surface of the
metal a cathode in relation to its
surroundings.
true
O Eror
10- Processes that include recycling
of materials are meant by the
processes in which raw materials
and by-products accompanying the
main product are recycled and
benefited from.
true
O Emor
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student weighed 10 pennies on a balance and recorded the following masses: 3.112 g, 3.109 g, 3.059g, 3.079 g, 3.025g 3.081 g, 2.504 g, 3.050 g, 3.072 g, 3.064g a)Are there outliers among the masses of pennies? b)Calculate the mean mass of pennies and the standard deviation,withthe outlier(s) excluded. c)Re-write the mean mass of pennies with the precisionindicatedarrow_forwardIn a particular trial, a student got a value for R of 0.083. Calculate the percent deviation of this result. Enter a number without the % sign. The percent deviation should be reported as a positive value.arrow_forwardwhat goes in the blue boxarrow_forward
- STARTING AMOUNT + X 7.6 ADD FACTOR x( ) (1)³ (100)³ Convert 7.6 cm³ to m³ 7.6 x 10-6 7.6 x 10-4 cm 1000 0.1 cm³ ANSWER (0.1)³ 100 m³ 1 (1000)³ m RESET J 0.01 (0.01)³ 0.076arrow_forwardDescribe how the confidence limit (uncertainty) of a measurement can be assessed and reported. Then write down an equation that describes how the measurement uncertainties of three measurements, p, q, and r, propagate into the uncertainty of x, where, r) and the standard deviations of p,q, and r, are expressed as $,,,, and s. If x=p2, what would the equation be? (15%)arrow_forwardIf an uncalibrated 5 (±0.03) mL pipet is used to deliver a total of 15 mL, what is the uncertainty delivered in the 15 mL?arrow_forward
- A calibrated pipet delivers a mean volume of 24.991 mL with a standard uncertainty of ± 0.006 mL. What is the uncertainty if you deliver four aliquots to reach 100 mL?arrow_forwardDensity of object analysis Dlameter (cm) Helght (cm) Mass (g) Volume (cm) Density (g/cm) 1.17 2.39 3.612 Object A Object B 1.19 2.10 3.306 Object C 1.15 1.63 2.367 Object D 1.14 1.72 2.471 Table view List view Density statistics Density (g/cm) Average Standard deviation Coefficient of variation (CV) True density 1.410 Absolute error % errorarrow_forwardexplain answer please for 4 & 5arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY