
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Using dimensional analysis, convert 17.2 moles of phosphorus into atoms. (1 mol = 6.02 x 10^23 atoms). Round to 3 significant figures
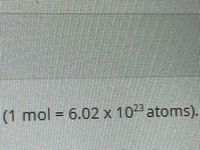
Transcribed Image Text:(1 mol =
6.02x10 atoms).
184
城
を
A 大
名古
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The chemical formula for hydrogen fluoride is HF. A chemist measured the amount of hydrogen fluoride produced during an experiment. She finds that 119. g of hydrogen fluoride is produced. Calculate the number of moles of hydrogen fluoride produced. Round your answer to 3 significant digits.arrow_forwardSuppose 5.71 g of potassium chloride is dissolved in 350. mL of a 64.0 m M aqueous solution of silver nitrate. Calculate the final molarity of chloride anion in the solution. You can assume the volume of the solution doesn't change when the potassium chloride is dissolved in it. Be sure your answer has the correct number of significant digits.arrow_forwardA chemist measures the amount of fluorine gas produced during an experiment. He finds that 3.1 g of fluorine gas is produced. Calculate the number of moles of fluorine gas produced. Round your answer to 2 significant digits. Ú mol 0 x10 Xarrow_forward
- A 0.732 g mixture of methane, CH4, and ethane, C2H6, is burned, yielding 2.046 g of CO2. What is the percentage by mass of CH4 in the mixture? Enter your answer with 2 significant digits.arrow_forwardWhat is the concentration, in units of grams of dye per liter of solution (g/L), of the dilute dye solution that is produced as follows? Transfer 16.51 mL of the stock solution (6.12 g/L) to a 200.0 mL volumetric flask and dilute to the 200.0 mL line with deionized water. Your answer should be reported to the correct number of significant figures.arrow_forwardA chemist measures the amount of hydrogen gas produced during an experiment. He finds that 575. g of hydrogen gas is produced. Calculate the number of moles of hydrogen gas produced. Round your answer to 3 significant digits. O mol x10 ?arrow_forward
- Determine the mass of the product from the following data. Mass Aluminum Foil (g) 2.5415 Mass Filter Paper (g) 0.2777 Mass Filter Paper + Product (g) 35.6484 Mass Product (g) Group of answer choices 35.3708 g 35.3705 g 35.3707 g 35.3709 g 35.3706 garrow_forwardPheromones are a special type of compound secreted by the females of many insect species to attract the males for mating. One pheromone has the molecular formula C₁9H38O. Normally, the amount of this pheromone secreted by a female insect is about -12 1.4 x 10 g. How many molecules does this quantity contain? Be sure your answer has the correct number of significant digits. moleculesarrow_forwardAqueous hydrobromic acid HBr will react with solid sodium hydroxide NaOH to produce aqueous sodium bromide NaBr and liquid water H2O .Suppose 34. g of hydrobromic acid is mixed with 8.90 g of sodium hydroxide. Calculate the minimum mass of hydrobromic acid that could be left over by the chemical reaction. Round your answer to 2 significant digits.arrow_forward
- Determine the mass of the product from the following data. Mass Aluminum Foil (g) 6.3284 Mass Filter Paper (g) 0.2489 Mass Filter Paper + Product (g) 86.2751 Mass Product (g) Group of answer choices 86.0263 g 86.0262 g 86.0260 g 86.0264 g 86.0261 garrow_forwardIf a chemist were given 2.99 grams of a mixture of sugar and sand which gave 1.35 grams of sand on analysis of the mixture, what percent by mass of sugar should the chemist report for the composition of the mixture?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY