
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
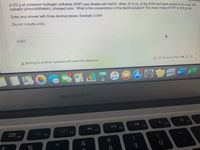
Transcribed Image Text:0.172 g of potassium hydrogen phthalate (KHP) was titrated with NaOH. When 31.5 mL of the KOH had been added to the acid. the
indicator (phenolphthalein), changed color. What is the concentration of the NaOH solution? The molar mass of KHP is 204 g/mol.
Enter your answer with three decimal places. Example: 0.544
Do not include units.
0.027
A Moving to another question will save this response.
Question 12 of 26
MacBook Air
F10
F9
F8
000
F7
F6
000 F4
F5
*
&
8.
9.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If 0.069 mol of salicylic acid was added to 62.92 mL of pentanol, what would be the concentration of the resulting solution in units of g solute/100 g solvent? MW of salicylic acid= 138.12 g/mol MW of pentanol= 88.15 g/mol Density of pentanol= 0.8144 g/mLarrow_forwardFor their first trial, a student pipettes 5.00 mL of a vinegar solution into an Erlenmeyer flask. The student titrates the vinegar with standardized NaOH with a molarity of 0.437 M. The volume of titrant used was recorded in the table above. Calculate the molarity of the vinegar solution for this trial. Enter a number without units.arrow_forwardA chemistry student weighs out 0.212 g of lactic acid (HC,H,03) into a 250. mL volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with 0.1000 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the equivalence point. Round your answer to 3 significant digits. mL ola Ox10 Ar ?arrow_forward
- Aa.23.arrow_forwardAn analytical chemist weighs out 0.138 g of an unknown monoprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. He then titrates this solution with 0.0600M NaOH solution. When the titration reaches the equivalence point, the chemist finds he has added 25.5 mL of NaOH solution. Calculate the molar mass of the unknown acid. Round your answer to 3 significant digits. 0 mol X S 629 OL Aarrow_forward3.45 mol of LiCl in 3.53 L of solution Express the molarity in moles per liter to three significant figures. 29.23 gC6H12O6 in 1.13 L of solution Express the molarity in moles per liter to three significant figures. 38.0 mgNaCl in 106.2 mL of solution Express the molarity in moles per liter to three significant figures.arrow_forward
- One way to determine the hardness of a water sample is to precipitate the calcium out of solution as a carbonate. The net ionic reaction for this precipitation is: Ca2+(aq) + CO32-(aq) → CaCO3 (s) A 1.00 L sample of water is drawn from a Phoenix, AZ well and 0.534 g of calcium carbonate is recovered using the above reaction. What was the Ca2+ concentration of the original water sample in ppm? Show your work and include units. Hint: ppm stands for parts per million, and in the case of an aqueous solution, this is equivalent to mg/L.arrow_forward5.00 ml of 7.35 M Fe(NO3)3 is combined with 1.00 mL of 0.50 M HCIO4 and 4.00 ml of 2.00 x 10-2 M KSCN. What is the concentration of Fe3+ in the solution after the other reactants are added? Enter your answer with two decimal places. Example: 1.32 Do not include units in your answer.arrow_forward- Part B The reaction described in Part A required 3.28 L of sodium chloride. What is the concentration of this sodium chloride solution? Express your answer with the appropriate units. View Available Hint(s) Value Units Submit When solutions of silver nitrate and sodium chloride are mixed, silver chloride precipitates out of solution according to the AgNO3 (aq) + NaCl(aq)→A£CI(s)+ NaNO3(aq) Part A What mass of silver chloride can be produced from 1.03 L of a 0.181 M solution of silver nitrate? Express your answer with the appropriate units. > View Available Hint(s) HA mass of AgCl = 873.9 Submit Previous Answersarrow_forward
- A chemist performs a gravimetric analysis. The chemist combines 1.00 L of 2.00 M AGNO, (ag) with 1.00 L of 4.00M NaCl (ag) in an Erlenmeyer flask. Both the AgNO3 (aq) solution and the NaCl(aq) solution are colorless. After the mixture has been stirred, a cloudy white substance is observed at the bottom of the flask. What is the expected mass of AgCl (s), in grams, assuming that the yield is 100%? In the box, enter the mass to the nearest gram.arrow_forwardYou perform a titration between Sodium hydroxide (NaOH) and Carbonic Acid (H₂CO3). The NaOH is in the burette and the H₂CO3 is in the Erlenmeyer Flask. 42.5 mL of 0.50 M NaOH was added to 25.6 mL of H₂CO3, what is the concentration of the H₂CO3? Give your answer to 2 decimal places..arrow_forward0.644 g of wood bleach, which contains oxalic acid, H2C2O4, was placed in an Erlenmeyer flask. The wood bleach was titrated with 23.57 mL of 0.379 M KOH solution. Determine the percent by mass of the H2C2O4 (a diprotic acid) in the sample of wood bleach. Enter your answer with the correct number of significant figures. If you do not enter the correct number of significant figures, your answer may be graded as incorrect even if it is otherwise correct.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY