
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
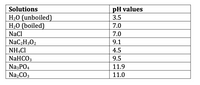
Transcribed Image Text:pH values
3.5
Solutions
H20 (unboiled)
H20 (boiled)
7.0
NaCl
7.0
NaC2H302
9.1
NHẠC1
4.5
NaHCO3
9.5
Na3PO4
11.9
Na2CO3
11.0
![Lab Report #4-2-3:
0.1 M NH4CI solution, using the given pH data, write expression for equilibrium
constant (Ka or Kb):
Ka = [NH3] [H*] / [NH4*]
Kb = [NH3] [H*] / [NH4*]
Ka = [NH4*] [OH"] / [NH4OH]
Kb = [NH4*] [OH"] / [NH4OH]](https://content.bartleby.com/qna-images/question/6a70e2d2-d641-435a-b212-d4266cff05fc/6f0345b7-d194-488d-a76c-8bd0aaf1ea17/a32kvei_thumbnail.png)
Transcribed Image Text:Lab Report #4-2-3:
0.1 M NH4CI solution, using the given pH data, write expression for equilibrium
constant (Ka or Kb):
Ka = [NH3] [H*] / [NH4*]
Kb = [NH3] [H*] / [NH4*]
Ka = [NH4*] [OH"] / [NH4OH]
Kb = [NH4*] [OH"] / [NH4OH]
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The pH of which of the following solutions will decrease by 1.00 pH unit when diluted with water to 10 times its original volume? 0.10 M HCI 0.10 M NAOH 0.10 M CH3COOH 0.10 MCH3COO Na" A mixture of 010 M CH;COOH and 0.10 M CH3COO'Na*arrow_forwardHelp neededarrow_forwardIn the laboratory, a general chemistry student measured the pH of a 0.558 M aqueous solution of isoquinoline, C9H-N to be 9.557. Use the information she obtained to determine the K, for this base. K(experiment) =arrow_forward
- For each of the salts (assume at this point completely soluble), write the equation for dissociation and label the ions as acidic, basic or neutral.Each salt listed will 100% dissociate NH4C2H3O2 Al2(CO3)3 Na2HPO4 NaI For each of the following salts, determine the pH of a 2.50M solution of the salt.You will need the Ka and Kb tables to complete this problem. NaBrO CH3NH3Cl NaClO2 C6H5OK CsNO3 C6H5NH3Brarrow_forwardexplain why the ph doesnt change significantly when a small amount of an acid or a base is added to a solution that contains equal amounts of the base NH3 and a salt of its conjugate acid NH4CLarrow_forwardThe pH of a 0.800 M aqueous solution of a weak acid is 1.99. What is the value of Ka for this acid?arrow_forward
- Be sure to answer all parts. For the titration of 40.0 mL of 0.150 M acetic acid with 0.100 M sodium hydroxide, determine the pH when: (a) 40.0 mL of base has been added. (b) 60.0 mL of base has been added. (c) 80.0 mL of base has been added.arrow_forwardhello i need help with this question The standard enthalpy of vaporization for C2F6 is 117.07 J/g at its normal boiling point of -78.20°C.Calculate the standard entropy of vaporization (ΔS°vap) of C2F6(l).Answer to the nearest 0.1 J K-1 mol-1.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY