
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
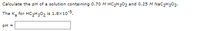
Transcribed Image Text:Calculate the pH of a solution containing 0.70 M HC2H3O2 and 0.25 M NaC2H302.
The K, for HC,H;0, is 1.8x10-5.
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution is prepared at 25 °C that Is initlally 0.43M in dimethylamine ((CH, NH, a weak base with K, =5.4x10, and 0.15M in dimethylammonlum -4 and 0.15M In dimethylammonium bromide ((CH,),NH,Br) ((CH,) NH,Br). Calculate the pH of the solution. Round your answer to 2 decimal places. bгomide 2. pH Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privac hparrow_forwardPlease help mearrow_forwardWhat is the pH of a solution that is 0.25 M CH3NH2 (methylamine) and 0.43 M CH3NH3Cl (methylammonium chloride)? (Assume Kw is 1.0 10-14.)arrow_forward
- If 0.50 L 0.20 M HF solution is mixed with solution A to make a mixed solution with a total volume of 1.00 L. Kw = 1.00x10-14; Ka(HF) = 6.6x10-4 at T=298K. (a) If solution A is 0.50 L 0.20 M HNO3, calculate the pH of the mixed solution. Ka(HNO3) = 100 (b) If solution A is 0.50 L 0.20 M NH4CI, calculate the pH of the mixed solution. Ko(NH3) = 1.8×10-5 %3D (c) If solution A is 0.50 L 0.20 M HNO2, calculate the pH of the mixed solution. Ka(HNO2) = 5.1×104 Hint: First list an equation that shows electrical neutrality in the solution. Also, neglect the [H*] contribution from water. Then use [H*] and the equilibrium constant(s) to express the anion concentrations in the equation you obtained for electrical neutrality, and you will find there is single unknown [H*] in the equation.arrow_forwardThe ionization constant of acetic acid HC2H3O2 is 1.8 x 10-5. What will be the pH of a 0.10 M HC2H3O2 solution which is 0.10 M in NaC2H3O2arrow_forward8:43 1 ll 5GE Question 11 of 25 Submit The pH for 0.0715 M solution of CCl,CO2H is 1.40. Determine the value of Ka for CCl,CO2H. 1 2 Based on the given values, fill in the ICE table to determine concentrations of all reactants and products. CCI,CO,H(+ H20(1) =H;0*(aq) +CCl,CO2 (ac Initial (M) Change (M) Equilibrium (M) 5 RESET 0.0715 1.40 -1.40 0.040 -0.040 0.032 -0.032 +x -X 0.0715 + x 0.0715 - 1.40 + x 1.40 - x 0.040 + x 0.0340 - x 0.032 + x 0.032 - xarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY